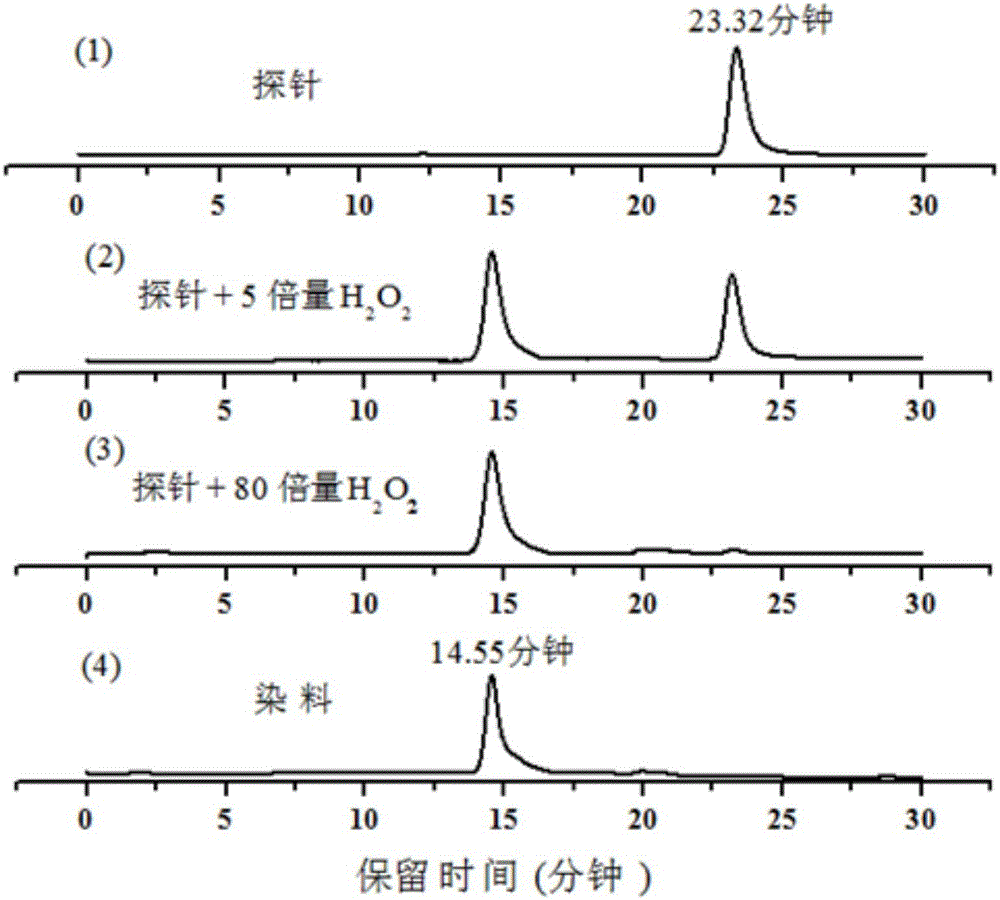
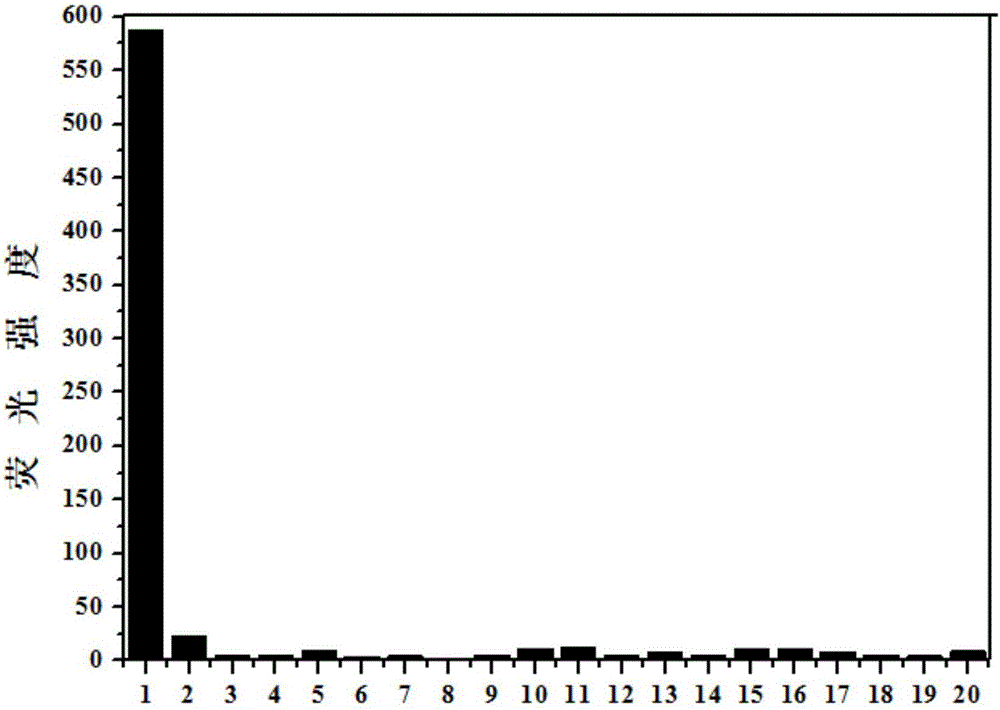
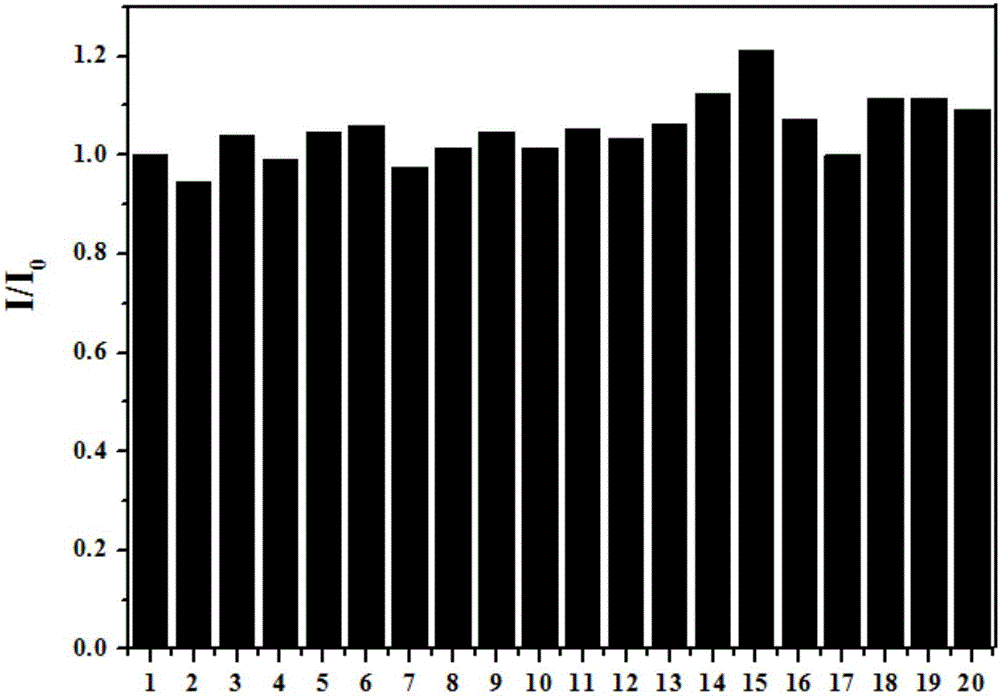
Synthesis and application of novel fluorescence probe for detecting hydrogen peroxide
A technology of hydrogen peroxide and fluorescent probes, applied in the field of chemical analysis and detection, to achieve the effects of high yield, large Stokes shift and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: the preparation of 3-hydroxyphthalonitrile
[0025] Take 3-nitrophthalonitrile (1.0g, 5.8mmol), potassium carbonate (0.9g, 6.35mmol) and sodium nitrite (0.4g, 5.8mmol) in a 25ml round bottom flask, add 15mL of dimethyl Dissolve in sulfoxide, heat the oil bath to 130°C, stir and reflux, analyze the progress of the reaction by TLC thin-layer chromatography, cool to room temperature after 30 minutes of reaction, slowly add distilled water (45mL) to dilute the reaction solution, dilute hydrochloric acid (2M) to adjust the pH to 3. The precipitate was precipitated, then filtered under reduced pressure, the filter cake was washed three times with distilled water, recrystallized in glacial acetic acid to obtain brown needle-shaped crystals, filtered by suction to obtain all crystals, and dried overnight in a vacuum desiccator to obtain the product 3- Hydroxyphthalonitrile. Yield: 0.51 g. Yield 60%.
Embodiment 2
[0026] Embodiment 2: the preparation of dyestuff
[0027] Take 3-hydroxyphthalonitrile (288mg, 2mmol), 2-aminopyridine (385mg, 4.1mmol) and calcium chloride (46mg, 0.41mmol) in a 25mL round bottom flask, add 6mL n-butanol to dissolve. Under the protection of argon, the oil bath was heated to 110°C and stirred and refluxed. TLC thin-layer chromatography was used to detect the reaction progress. After 5 days, the reaction was completed. The reaction solution was cooled to room temperature, and the solvent was removed by distillation under reduced pressure. The cake was placed in a vacuum desiccator overnight, further purified by column chromatography, and the obtained orange powder was dried in vacuum overnight. Yield: 150.8 mg. Yield 26.6%. 1 H NMR (400MHz, CDCl 3 )δ H 13.70(s, 1H), 8.62(m, 2H), 7.78(t, J=7.9Hz, 2H), 7.60(s, 1H), 7.52(d, J=7.3Hz, 2H), 7.38(d, J =8.0Hz, 1H), 7.19-7.09(m, 3H). 13 C NMR (101MHz, CDCl 3 )δ c 160.10, 159.34, 155.81, 155.59, 153.70, 147.98,...
Embodiment 3
[0028] Embodiment 3: the preparation of probe molecule
[0029] Take the dye (48mg, 0.15mmol) obtained in the previous step, potassium carbonate (400mg, 3mmol), potassium iodide (80mg, 0.5mmol) in a 100mL round bottom flask, add 30mL acetonitrile to dissolve, stir at room temperature for 30 minutes, then add 4-bromomethyl Phenylboronic acid pinacol ester (47.6mg, 1.6mmol), then the oil bath was heated to 61°C and stirred and refluxed, TLC thin layer chromatography was used to detect the progress of the reaction, the reaction was completed after 30 minutes, the reaction solution was cooled to room temperature, and removed by rotary evaporation under reduced pressure. The solvent was acetonitrile, and purified by flash column chromatography to obtain a yellow powder. Yield: 59 mg. Yield: 73.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com