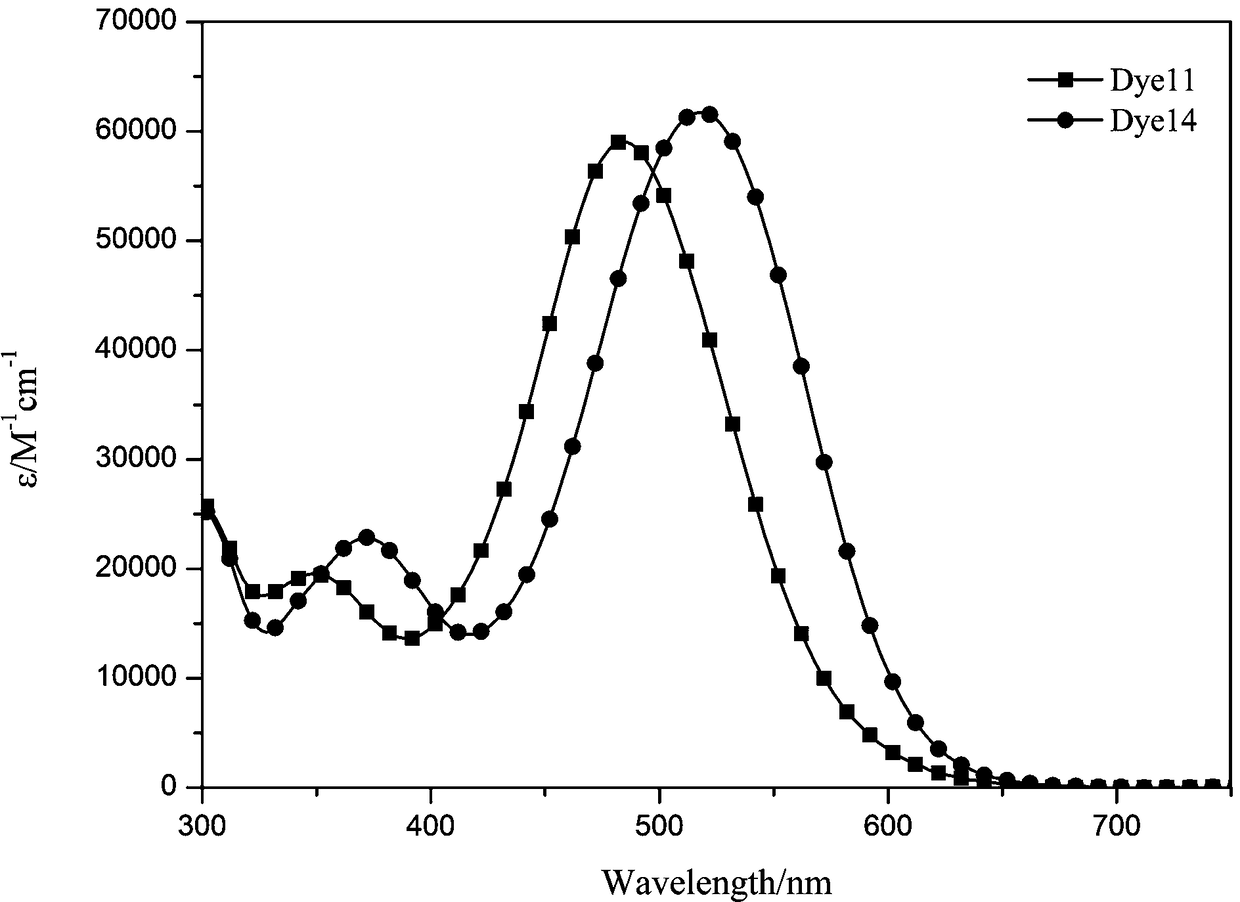
Organic dyes based on unsymmetrical trithienopyrrole and its preparation method and application
A phenopyrrole and organic dye technology, applied in the field of asymmetric trithienopyrrole-based organic dyes and its preparation, can solve problems such as expensive preparation costs, environmental pollution restrictions, complex synthesis and separation processes, and achieve improved efficiency, electronic The effect of high cloud density and broadened absorption spectrum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the preparation of organic dye 11
[0042] The synthetic route is as follows:
[0043]
[0044] The raw material 1 used in this example was prepared according to the literature Q.Yu, W.Fu, J.Wan, X.Wu, M.Shi, H.Chen.ACSAppl.Mater.Interfaces, 2014, 6, 5798-5809; 9. 4-hexyloxyaniline and 3-bromo-2-thiophene zinc bromide According to literature Z.Wang, M.Liang, Y.Tan, L.Ouyang, Z.Sun, S.Xue.J.Mater.Chem .A, 2015, 3, 4865–4874, and other reagents can be obtained commercially.
[0045] Synthesis of Intermediate 2:
[0046] Into a 100mL single-necked round-bottom flask, add 3.75g of raw material 1 and 20mL of N-N dimethyldimethylformamide, then add 2.13g of N-bromosuccinimide to the system in batches, and keep the Light reaction for 8 hours; quenched with water, extracted with ethyl acetate, dried over anhydrous magnesium sulfate, and evaporated under reduced pressure to remove the solvent; the crude product was subjected to column chromatography (eluent: ...
Embodiment 2
[0063] Embodiment 2: the synthesis of organic dye 14
[0064] The synthetic route is as follows:
[0065]
[0066] Raw material 9 and intermediate 6 were synthesized by referring to the preparation method in Example 1, and other reagents can be purchased commercially.
[0067] Synthesis of intermediate 12:
[0068] Under the protection of argon, in a 100mL two-necked round-bottomed flask, 0.495g of intermediate 6 and 10mL of anhydrous DMF were added successively, and then 0.187g of N-bromosuccinimide was added in batches to the reaction solution. The reaction system was React at 25°C in the dark for 8 hours; then add 0.613g of phosphorus oxychloride water to the reaction solution, and continue the reaction at 25°C for 4h; add ice water to quench the reaction, extract with ethyl acetate, dry over anhydrous magnesium sulfate, and concentrate by rotary evaporation. The product was purified by column chromatography (eluent: petroleum ether / dichloromethane = 5 / 1-1 / 1) to obtain...
Embodiment 3
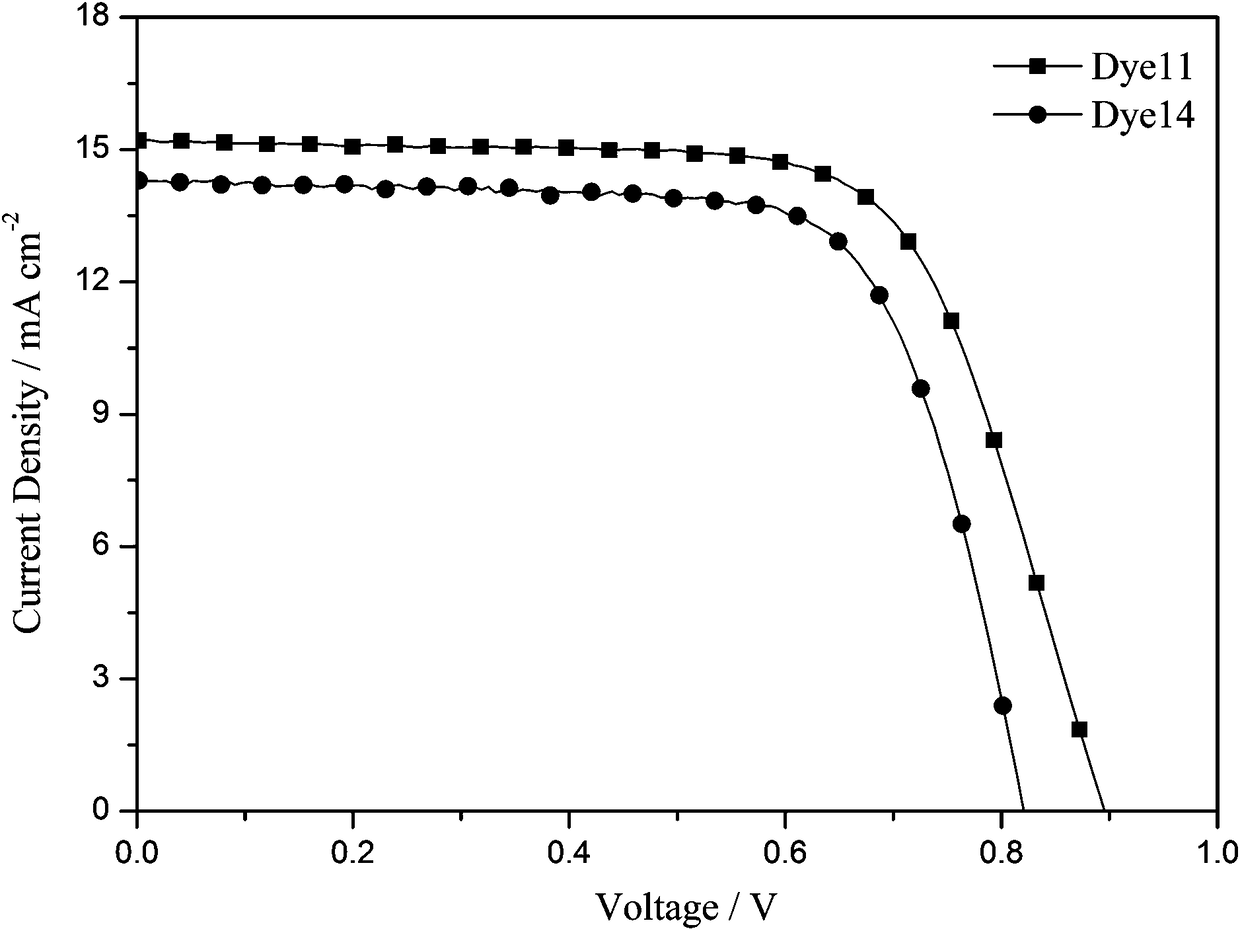
[0074] Application of the dye 11 prepared in Example 1 in the preparation of dye-sensitized solar cells. Its specific use method is the same as the use method disclosed in the invention patent application with the application number 201510015966.7 and the invention title "A Carbazole-type Photosensitizing Dye and Its Preparation Method and Application". Test light source: AM 1.5 (solar simulator-Oriel 91160-1000, 300W), data acquisition using Keithley 2400 digital source meter. see test results image 3 , the open circuit voltage of the battery (V oc ) is 885mV, short-circuit current density (J sc ) is 15.2mA cm -2 , the fill factor (FF) is 0.68, and the photoelectric conversion efficiency is 9.22%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| open-circuit voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com