Cinnamic acid derivative with aldose reductase inhibitory activity as well as preparation method and application thereof
A cinnamic acid derivative and a technology for inhibiting activity are used in the preparation of medicines for treating diabetic complications and diseases caused by oxidative stress. exact question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
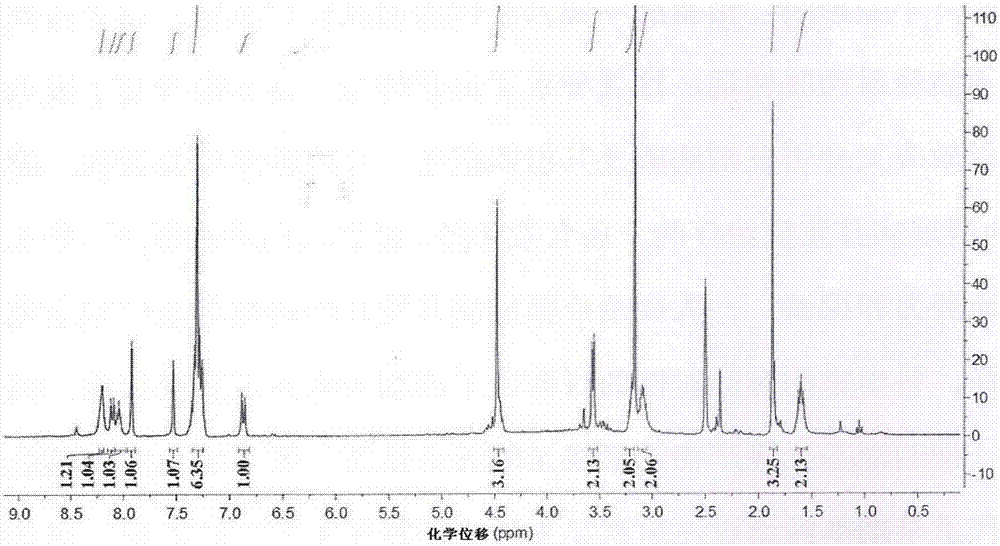
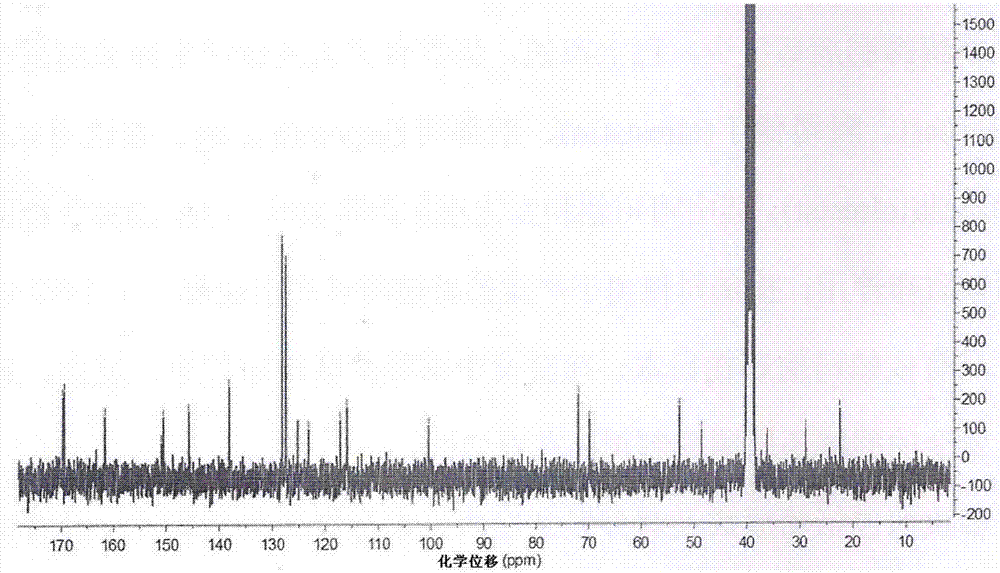
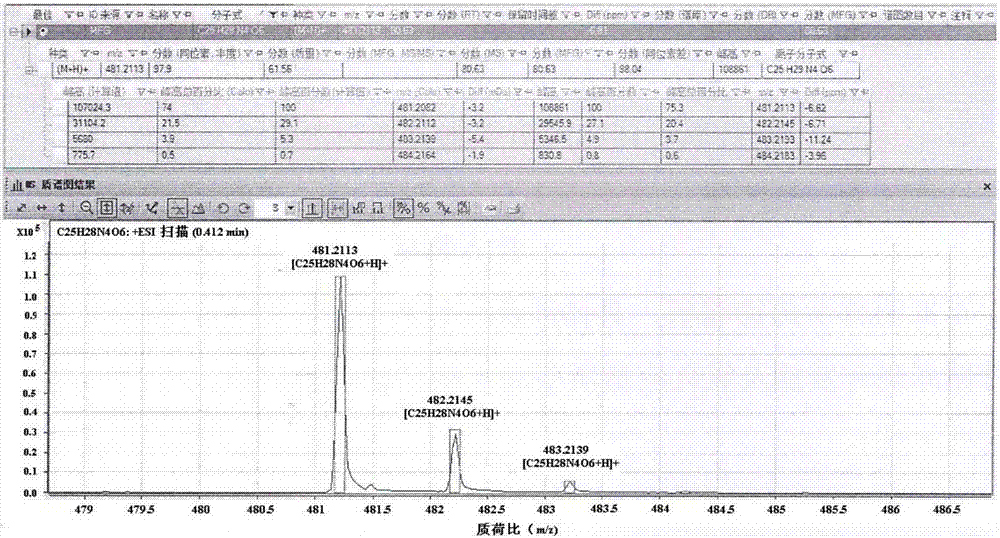
[0076] Example 1: Preparation of novel cinnamic acid derivatives with aldose reductase inhibitory activity
[0077] 1.1. Preparation of 3-N-(N-acetyl-O-benzyl-D-seryl)-α-cyano-3,4-dihydroxycinnamoylpropylenediamide
[0078] Include the following five steps:
[0079] (1) Synthesis of compound α-cyano-3,4-dihydroxybenzoic acid: Weigh 138mg (1.0mmol) of 3,4-dihydroxybenzaldehyde in a dry 50mL round bottom flask, add cyanoacetic acid 85mg ( 1.0mmol), ammonium acetate 15.4mg (0.2mmol), syringe weighed glacial acetic acid 48mg (0.8mmol) into the reaction system, then added 10mL of toluene as a solvent, the reaction bottle was connected to a small water separator, condenser tube, drying tube. Stir and reflux the reaction in an oil bath at 120°C for 18h. After the reaction was completed, it was lowered to room temperature, then stood at low temperature, filtered, and the filter cake was washed three times with dichloromethane (DCM) to obtain a yellow solid with a yield of 93.2%. 1 ...
Embodiment 2
[0158] Embodiment 2: the inhibitory activity of the synthetic compound of embodiment 1 to aldose reductase enzyme
[0159] In this example, aldose reductase (AR), reduced coenzyme II (NADPH), and DL-glyceraldehyde were purchased from Sigma; Epalrestat was purchased from Tokyo Chemical Industry Co., Ltd.
[0160] Experimental procedure: the enzyme reaction system was carried out on a 96-well plate, and the reaction system included: 100 μL of PBS buffer (pH=6.2), 20 μL of 1.5 mmol / L NADPH, 20 μL of 100 mmol / L DL-glyceraldehyde, different concentrations (Epalrestat or tested sample) solution 20 μL, AR diluent 20 μL, distilled water 20 μL. The experimental setup blank photo group, standard control group and enzyme metabolism group are shown in Table 2-1.
[0161] Table 2-1. Table of grouping and administration
[0162]
[0163] Note: All volume units above are in μL.
[0164] After incubating the above liquid at 37°C for 5 minutes, except for the blank solvent group, each gr...
Embodiment 3
[0172] Embodiment 3: the in vitro antioxidant activity of the compound synthesized in embodiment 1
[0173] In this example, Trolox (6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid); 1,1-diphenyl-2-picrylhydrazyl (DPPH) were purchased from Sigma.
[0174] Experimental procedure: the reaction system was carried out on a 96-well plate, 40 μL of each test solution was added into the 96-well plate, and then 160 μL of DPPH solution was added in parallel to each well. At the same time, a control group (40 μL methanol + 160 μL DPPH) and a blank group (40 μL test substance + 160 μL methanol) were set up. Shake for 1 min to make it evenly mixed, place the 96-well plate in the dark for 0.5 h, and quickly put the plate into a microplate reader with a detection wavelength of 517 nm. The final absorbance (A) value was measured, each sample was detected three times in parallel, and the average value was taken. The formula for calculating the clearance rate is as follows:
[0175] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com