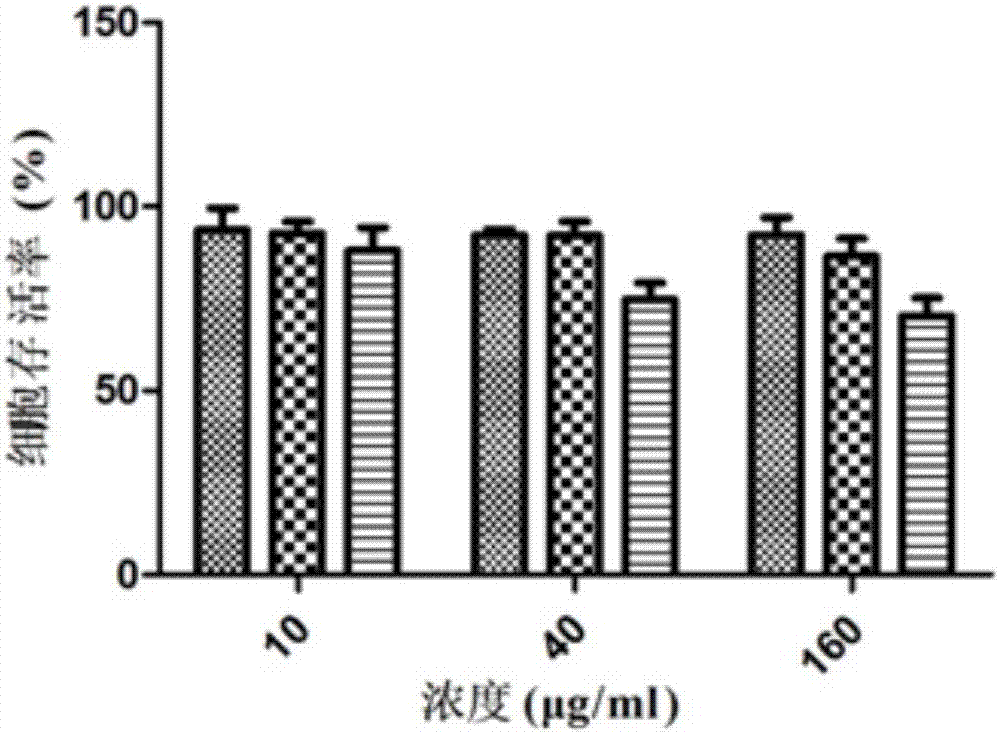
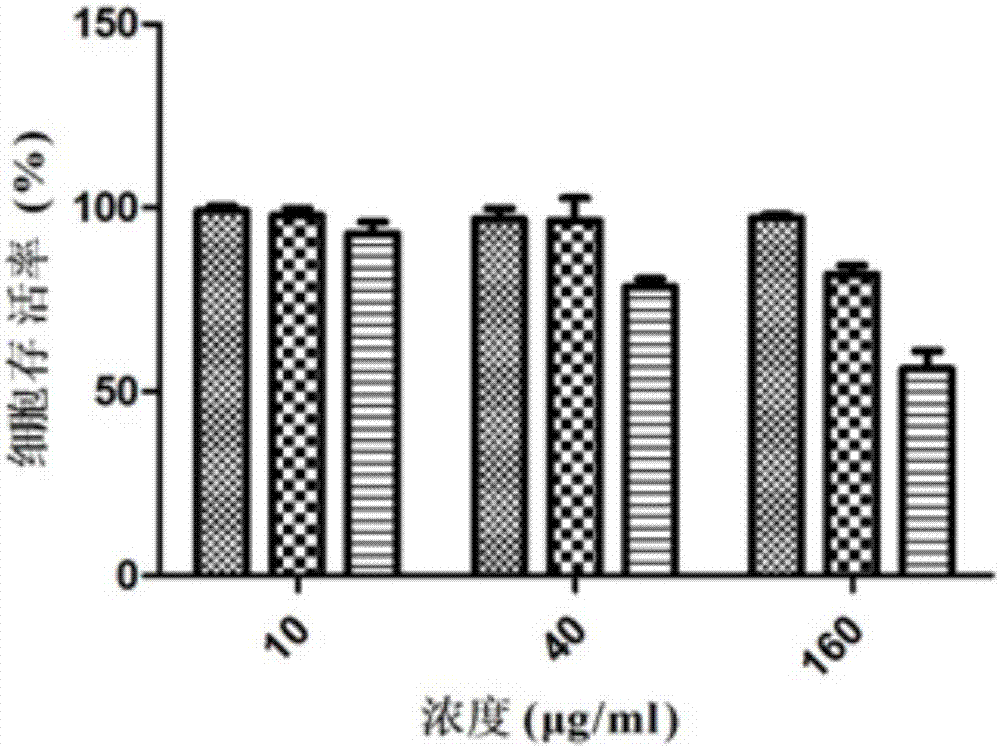
Antioxidative small peptides for inhibiting melanin production and preparation method and application thereof
A technology of inhibiting melanin and anti-oxidation, which is applied to the preparation method of peptides, chemical instruments and methods, and cosmetic preparations, etc. It can solve the problems of poor light absorption and unsuitable for daytime use, slow tyrosinase inhibitory effect, and unstable vitamin C. problem, achieve the effect of no hemolytic activity, no cytotoxicity, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Chemical synthesis of OGOT, Magin-FL and Ansin-FL:
[0022] (1) Chemical synthesis of OGOT, Magin-FL and Ansin-FL: According to the amino acid sequence of the short peptide, its full sequence was synthesized with an automatic peptide synthesizer (433A, Applied Biosystems), and desalted by HPLC reverse-phase column chromatography.
[0023] (2) Molecular weight was determined by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF).
[0024] (3) The purity of the purified OGOT, Magin-FL and Ansin-FL was identified by high performance liquid chromatography (HPLC), the molecular weight was determined by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF), and the isoelectric point was determined by isoelectric focusing electrophoresis , Determining the amino acid sequence structure with an automatic amino acid sequencer.
[0025] OGOT, Magin-FL and Ansin-FL are straight-chain short peptides. Among them...
Embodiment 2
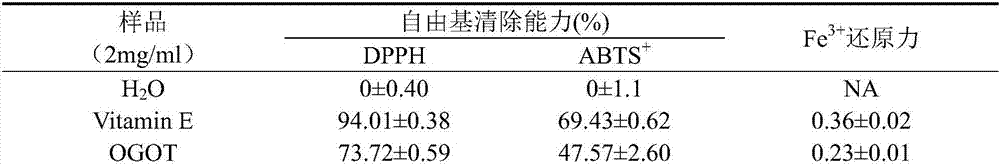
[0027] Antioxidant activity assay of OGOT, Magin-FL and Ansin-FL:
[0028] (1) DPPH radical scavenging activity (DPPH radical scavenging assay)
[0029] Weigh a certain amount of DPPH (2,2-diphenyl-1-picrylhydrazyl hydrate, Sigma, USA), dissolve it in methanol, and make 6×10 -5 The solution of M is prepared and used immediately. Mix 48 μl of DPPH solution with 2 μl of sample (2 mg / ml) (the mass ratio of final sample to DPPH is 3:1), let it stand in the dark for 30 minutes at room temperature, and measure the absorbance at 517 nm. In the blank control group, the sample to be tested was replaced by the sample dissolution medium. The experiment was done in triplicate, and methanol was used when the UV spectrophotometer was zeroed.
[0030] DPPH·clearance rate (%)=(AB-AA) / A B×100 (AB: absorbance value of blank control group; AA: absorbance value of sample group).
[0031] (2)ABTS ·+ Free radical cation scavenging activity
[0032] ABTS (3-ethylbezothiazoline-6-sulfonic acid)...
Embodiment 3
[0041] Tyrosinase half inhibitory concentration IC 50 Determination of value:
[0042] The tyrosinase activity inhibition experiment uses L-DOPA as the reaction substrate, and detects the absorbance at 475nm of the substrate colored substance dopaquinone catalyzed by tyrosinase, which indirectly reflects the inhibitory activity of the sample on tyrosinase.
[0043] Using a 100 μL reaction system, successively add phosphate buffer, samples of different concentrations (10, 25, 50, 100, 200 μM) and mushroom tyrosinase, and then incubate at 37°C for 10min, then add the substrate, and incubate at 37°C After reacting for 10 minutes, measure the OD value at a wavelength of 475nm. Calculate the relative activity inhibition rate of mushroom tyrosinase according to the measured OD value: inhibition rate (%)=(1-OD value of experimental group / OD value of control group)×100%.
[0044] The data were calculated using SPSS software to calculate the half-inhibition concentration IC of each s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com