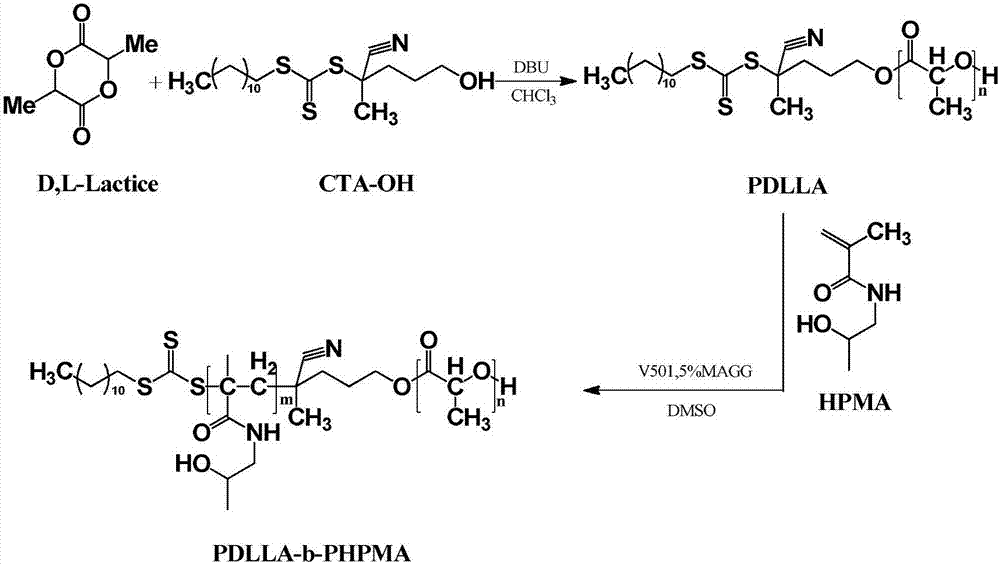
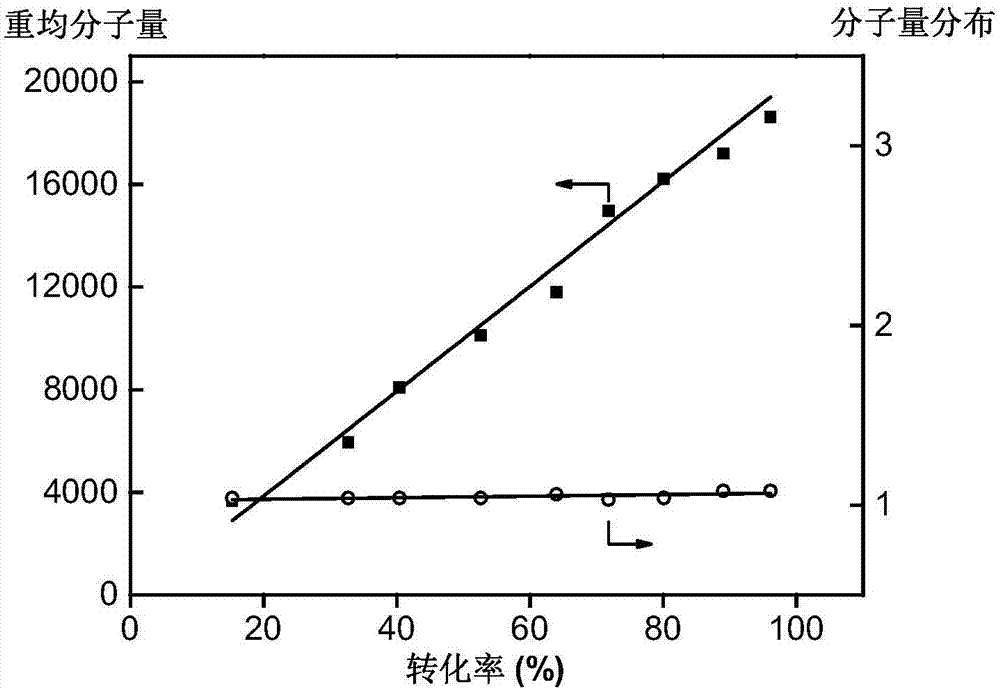
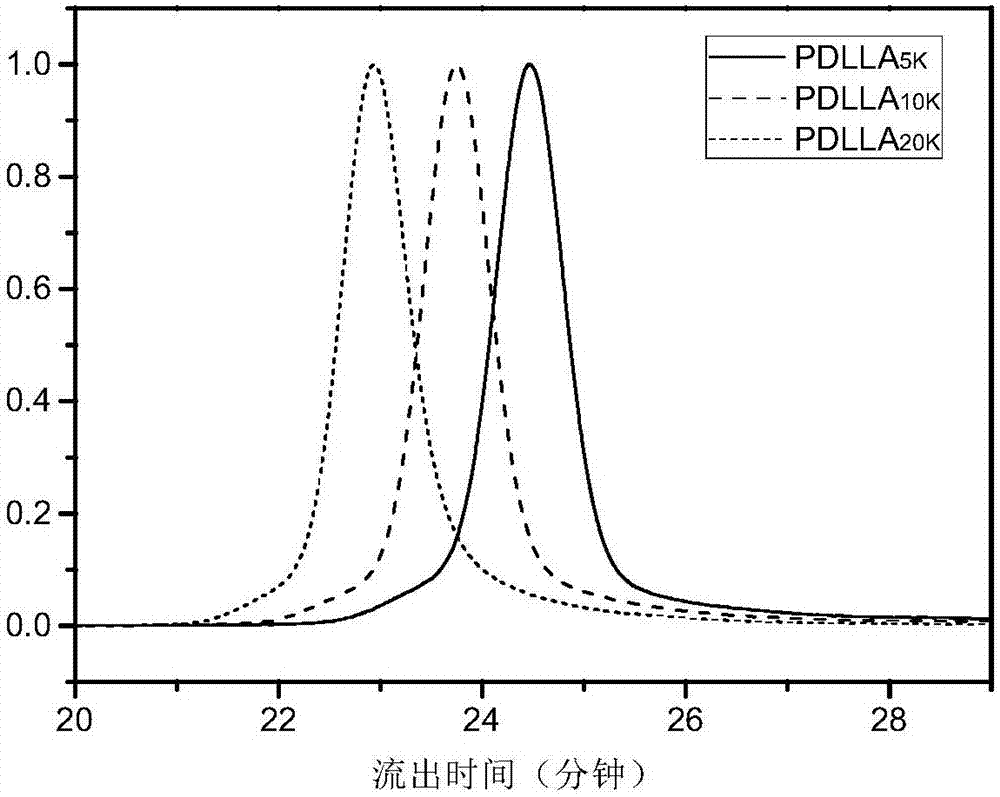
Block polymer, drug carrier containing same, and preparation method and application of block polymer
A technology of block polymers and polymers, which is applied in the direction of medical preparations, drug combinations, and pharmaceutical formulations of non-active ingredients, and can solve the problems of EPR effect drug release, wide molecular weight distribution, and uncontrollable polymer molecular weight, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] The synthesis of embodiment 1,4-dodecyl trithioester group-4-cyanopentanol (CTA-OH)
[0065]
[0066] Take a 1000mL round bottom flask, add 14.6gKOH into 450mL water, then slowly add 40.4g (48mL) dodecyl mercaptan to obtain an emulsified liquid solution, and slowly add 0.8g methyl trioctylammonium chloride and 14.4g ( 12.06mL) CS 2The mixed solution was observed to change from earthy yellow to yellow and finally to orange. After reacting at room temperature for 1 hour, cool to -5°C in an ice-salt bath (NaCl), slowly add 20 g of p-toluenesulfonyl chloride, keep the reaction at -5°C for 2 hours, then react in a water bath at 45°C for 45 minutes to form an orange oily product. Cool in an ice-water bath for 1 hour to form a solid product, which was filtered with suction and washed several times with ice water to remove water-soluble impurities. Then recrystallize and purify in acetone: gradually add acetone in a water bath at 61°C, and stir continuously until the solut...
Embodiment 2
[0069] Embodiment 2, the synthesis of ethylene glycol single 4-dodecyl trithioester group-4-cyanovalerate
[0070]
[0071] Take 5 g of didodecyl bis-trithiocarbonate prepared in Example 1 and 5 g of 4,4′-azobis(4-cyanovaleric acid) and dissolve them in 150 mL of ethyl acetate, reflux and react in the dark for 24 hours , the reaction temperature was 95° C., and the obtained final product was separated, purified and recovered by column chromatography again, and the mobile phase was petroleum ether: ethyl acetate = 2:1. Gained product 4.03g and 3.1g ethylene glycol are dissolved in 50mL anhydrous dichloromethane, add 4.5g dicyclohexylcarbodiimide (DCC) and 0.2g4-dimethylaminopyridine (DMAP) to catalyze and carry out esterification reaction, reaction After 48 hours, the produced by-product dicyclohexylurea was removed by filtration, and the obtained final product was separated, purified and recovered by column chromatography again, and the mobile phase was petroleum ether:ethy...
Embodiment 3
[0072] Embodiment 3, the synthesis of ethylene glycol mono-2-bromoisobutyrate
[0073]
[0074] Dissolve 9.3g of ethylene glycol and 2.02g of triethylamine in 100mL of dichloromethane, use an ice-water bath to lower the temperature of the system to 5°C, and slowly add 4.6g of 2-bromoisobutyryl bromide dropwise using a constant pressure dropping funnel. React for 24 hours after completion. After the reaction was completed, the generated triethylamine hydrobromide was removed by filtration, and the filtrate was washed three times with 1% sodium bicarbonate aqueous solution and pure water respectively. The collected organic phase was dried by adding anhydrous sodium sulfate, and then concentrated by rotary evaporation to remove the organic solvent to obtain a pure product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com