Oligopolyphenylene vinyl compound as well as preparation method and application thereof
A polyphenylene vinylene and compound technology, which is applied in the field of oligopolyphenylene vinylene compounds and their preparation, can solve problems such as side effects of medication, killing of tumor cells, and affecting the survival period of patients.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0093] In the present invention, the paclitaxel modified with acryloyl chloride is preferably paclitaxel whose 2'-hydroxyl group is modified with acryloyl chloride. The acryloyl chloride-modified paclitaxel can be prepared by conventional chemical methods. In a preferred case, the preparation method of the modified paclitaxel comprises: contacting paclitaxel with acryloyl chloride in an inert atmosphere. Wherein, the dosage ratio of the paclitaxel to the acryloyl chloride is 1:1-2, preferably 1:1-1.5. The contact solvent is dichloromethane. The contacting conditions are as follows: the temperature is -20-60°C, preferably -10-10°C; the time is 1-24 hours, preferably 2-4 hours. The contacting also includes: washing, extracting, drying, solvent evaporation and separation of the product in sequence. Wherein, the washing includes the process of washing with saturated aqueous sodium bicarbonate solution and deionized water; the extraction is carried out with dichloromethane; the ...
Embodiment 1
[0113] This example is used to illustrate the preparation method of compounds with the structures shown in formulas (1)-(5).
[0114] (1) compound shown in preparation formula (8) and formula (9)
[0115] 2.31g of the compound shown in formula (6), 3.67g of 1,2-dibromoethane, 1.38g of potassium carbonate and 2.64g of 18-crown ether-6 were dissolved in 60mL of acetone, and a catalytic amount of potassium iodide was added, stirred in an ice bath Oxygen was removed under nitrogen protection and reacted at 75°C for 24 hours. After the mixture was cooled to room temperature (25°C), it was washed once with 1 mL of saturated aqueous sodium bicarbonate solution and twice with 2 mL of deionized water, and 10 mL of dichloromethane was added to extract the product. The organic phase was dried with anhydrous magnesium sulfate, evaporated to After the solvent was separated by silica gel column (eluent: petroleum ether: ethyl acetate: dichloromethane = 10:2:1 (volume ratio)), 1.81 g of whi...
Embodiment 2
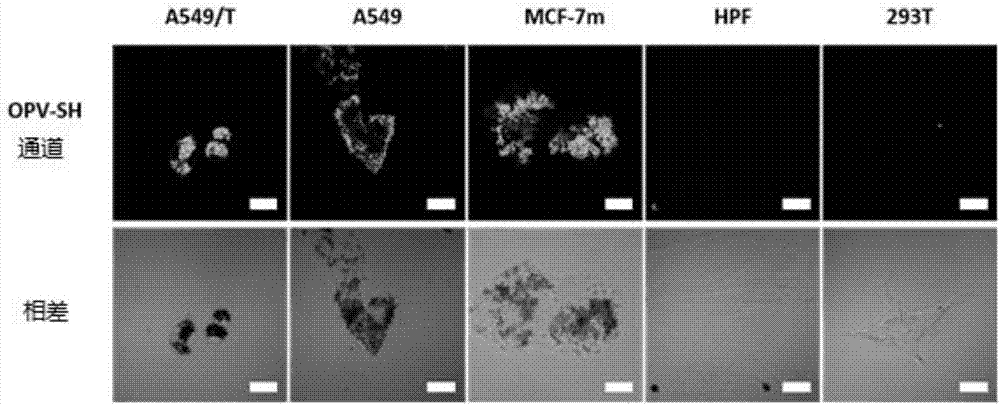
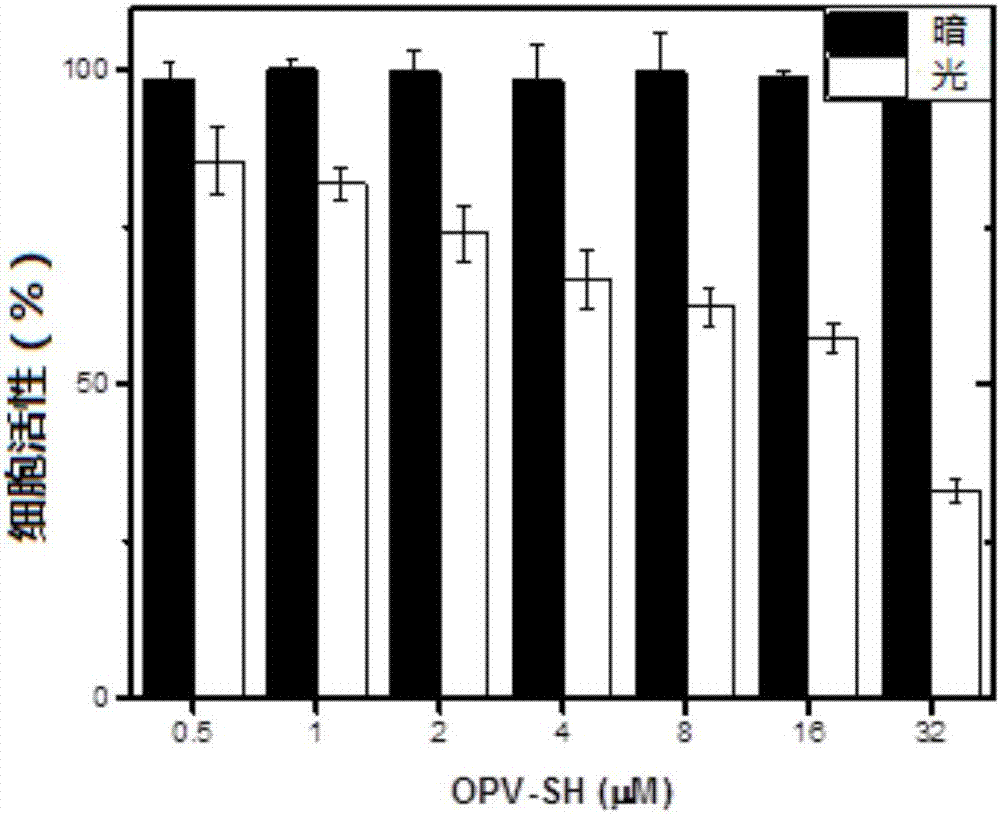
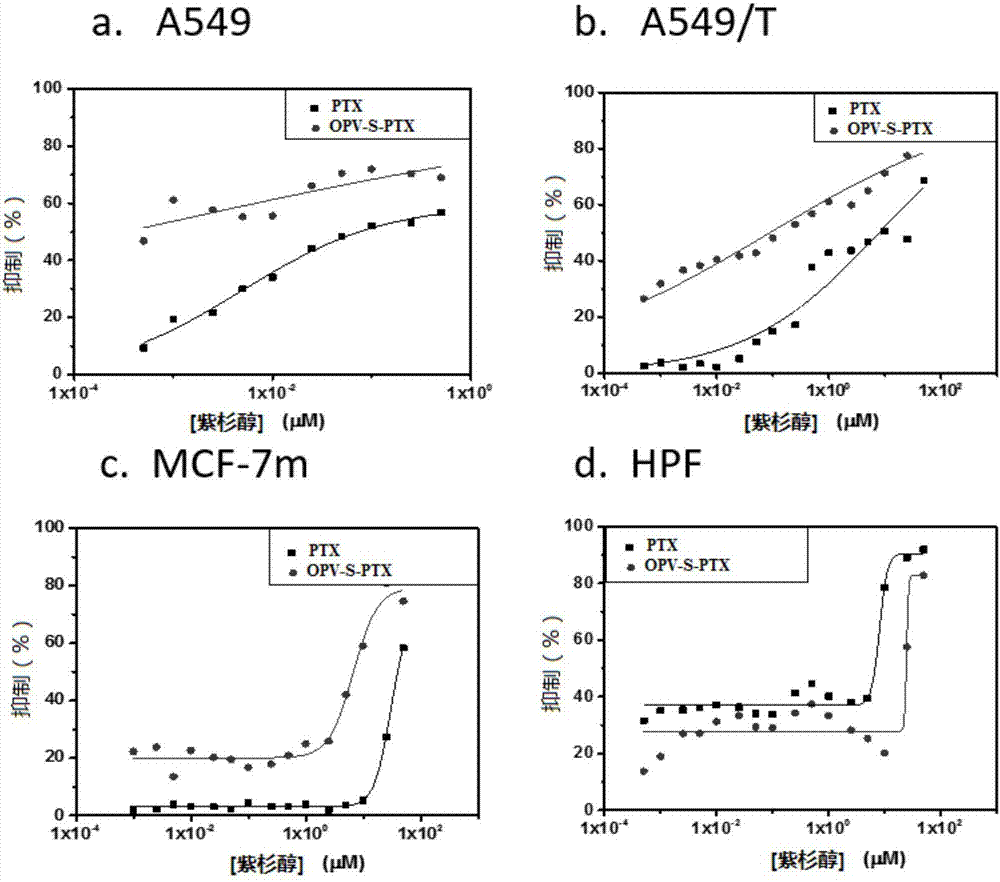
[0143] This example is used to illustrate the application of the OPV-SH compound of the present invention in the preparation of preparations for cell imaging.
[0144] Cell imaging experiments were performed using the OPV-SH obtained in Example 1, and characterized by a Confocal laser scanning microscope (FV1000-IX81, Olympus, Japan).
[0145] The specific steps are: Human lung cancer cell A549 is cultivated in a glass-bottomed culture dish with DMEM cell culture medium containing 10% serum for 24 hours. When the number of cells reaches nearly 60, add 2.5 micromoles of OPV-SH compound and continue to cultivate for 4 hours. , and set the control dish at the same time. The culture solution was removed and 100 nanomole of lysosome fluorescent dye (Lysotracker DND-99, Invitrogen) preheated at 37°C was added, and stained for 20 minutes. After staining, remove the culture medium carefully, wash with PBS three times, add PBS solution with a mass concentration of 4% paraformaldehyde,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com