Method for full-automatically synthesizing <18>F-marked pyrimidine acrylamide based EGFR positive-electron tracer agent through one-step method
A technology of pyrimidine acrylamide and positron tracer, which is applied in the field of pyrimidine acrylamide EGFR positron tracer and its preparation, can solve the problems of large influence of composition and experimental conditions, complicated operation, expensive instruments, etc., and achieve Overcoming the long synthesis time, simplifying the synthesis process, and shortening the synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1E
[0067] Embodiment 1EGFR TKI-BF 3 - Preparation of AZD9291
[0068] The BF 3 -The molecular structural formula of AZD9291 is as shown in formula I:
[0069]
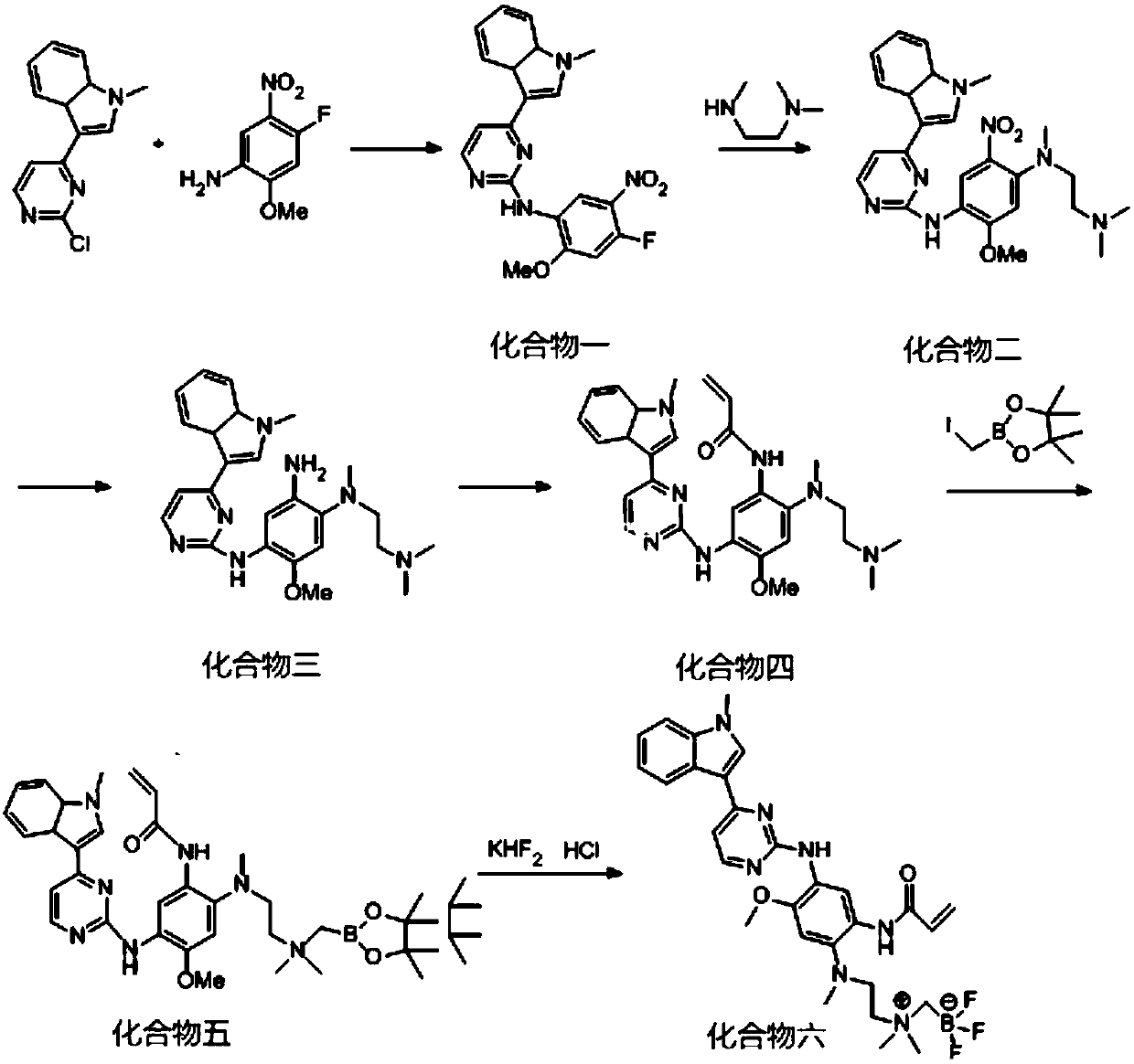
[0070] The preparation flow chart is as figure 1 As shown, the specific preparation method is as follows:
[0071] (1) Under nitrogen protection, 50g (2-chloropyrimidin-4-yl)-1-methyl-3a, 7a-dihydro-1H-indole, 38.03g 4-fluoro-2-methoxy-5- Nitroaniline and 37.09g p-toluenesulfonic acid were mixed in 580ml 2-pentanol, stirred mechanically at 105°C for 2.5h, cooled to room temperature, filtered with suction, the filter cake was washed with 2-pentanol, and dried to obtain a light brown solid , separated and purified by column chromatography, and eluted with an eluent obtained by mixing methanol and dichloromethane according to a volume ratio of 1:100, and finally obtained a light yellow needle-shaped solid, namely compound 1, with a yield of 85%;
[0072] (2) Mix 65g of compound 1, 19.65g of N,N,N'-trimethylethylenedi...
Embodiment 2
[0078] Embodiment 2 one-step synthesis 18 F-labeled pyrimidine acrylamide EGFR positron tracer ( 18 F-BF 3 -AZD9291)
[0079] Flowchart such as Figure 5 As shown, the synthesis steps are as follows:
[0080] 1. Will be transmitted from the medical cyclotron 18 F ions and water pass to the receiving bottle (1-4Ci), and are introduced into the anion exchange column QMA;
[0081] 2. Using 0.15mL K 2 CO 3 (0.9mg)+0.5mL acetonitrile was eluted from the QMA column 18 F ions are sent to the reaction bottle;
[0082] 3. Dissolve 3 mg of the precursor (prepared in Example 1) in 0.5 mL of 50% (v / v) DMF aqueous solution, add o-diazepine hydrochloric acid buffer solution to make the solution pH = 2, and add the mixed solution to the reaction bottle; heated in a water bath at 80°C for 15 minutes under the protection of nitrogen or helium;
[0083]
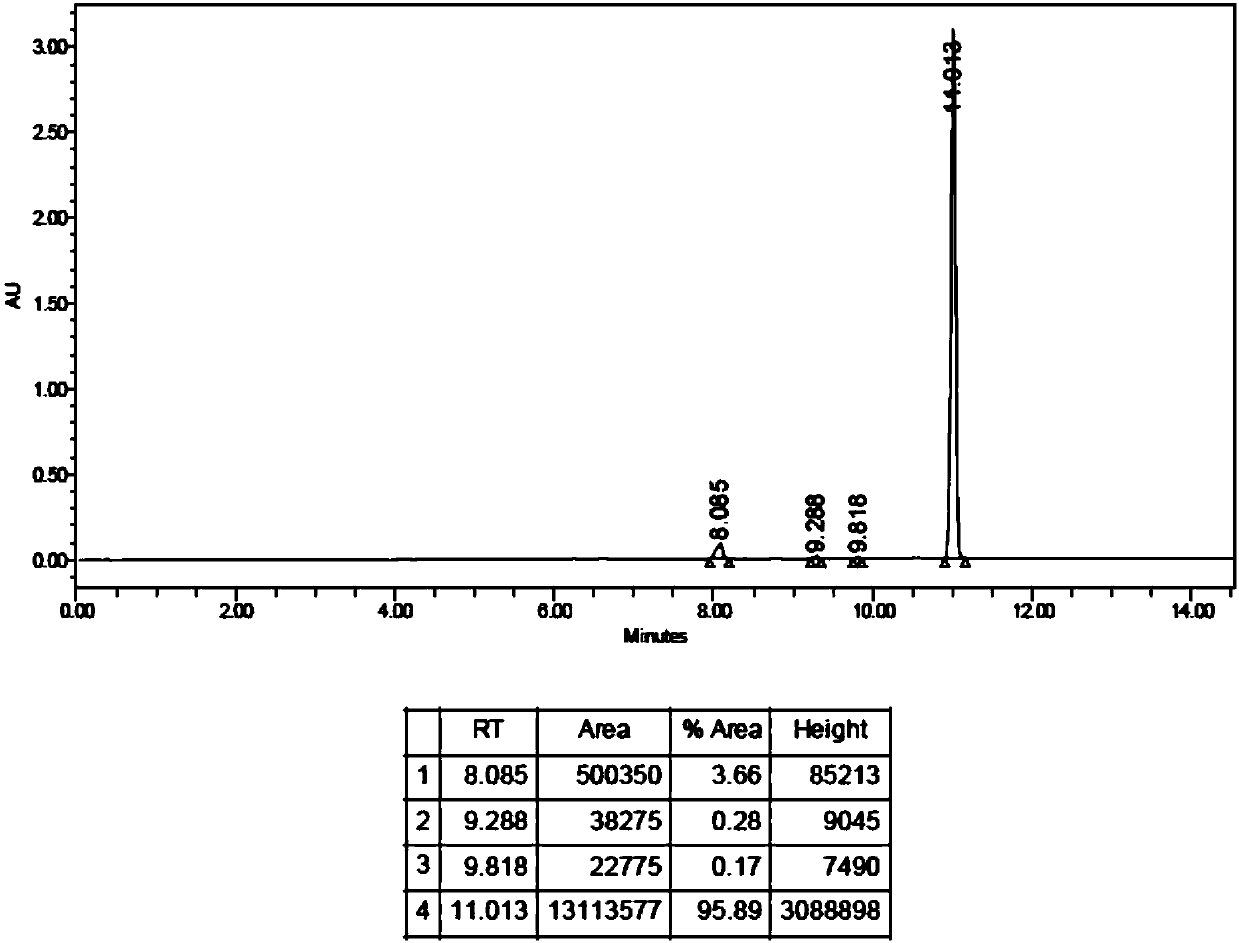
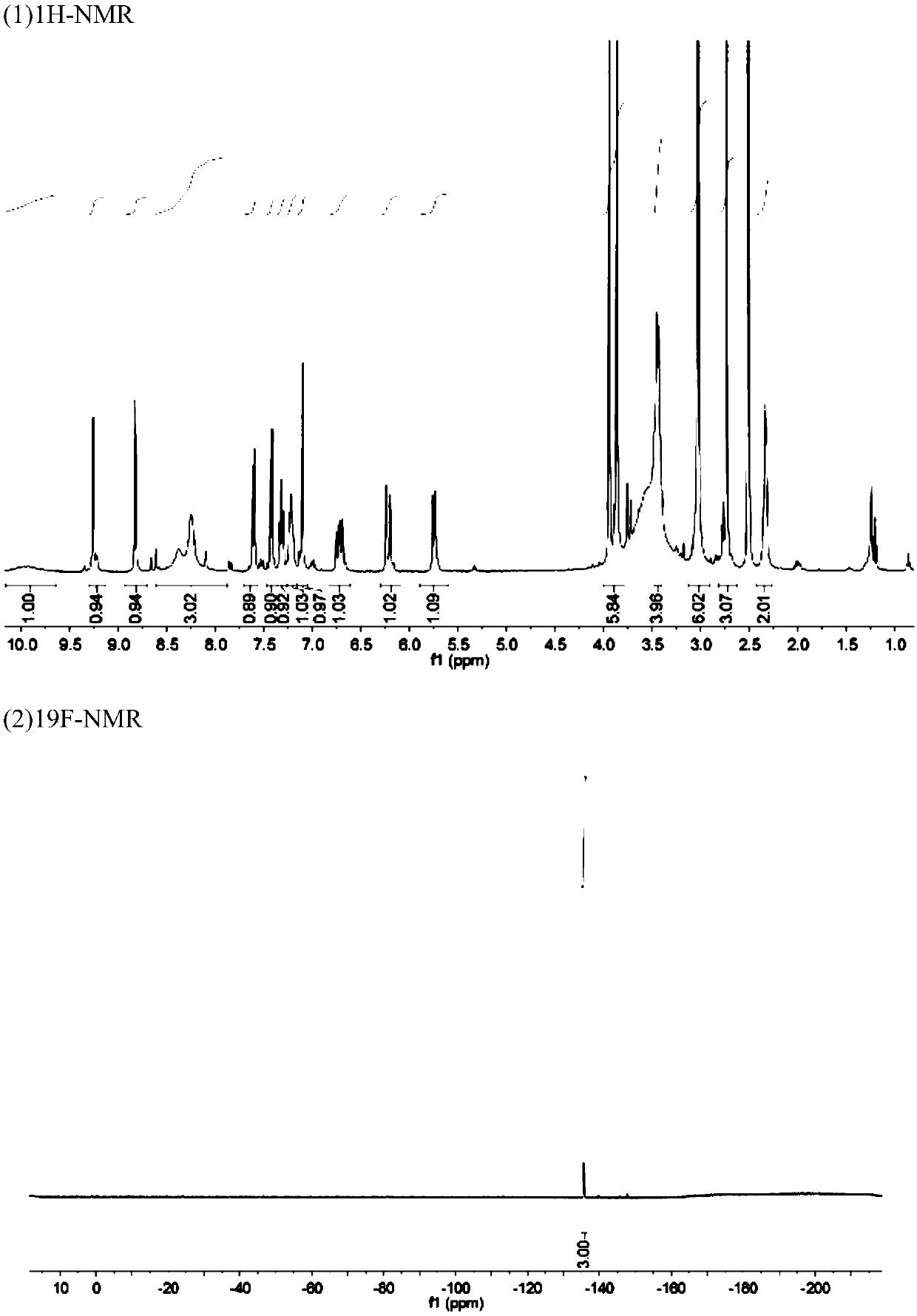
[0084] 4. Cool the temperature to 25°C, then pass the reaction solution through a C18 column to separate and purify the product,...
Embodiment 3
[0085] Embodiment 3 adopts one-step synthesis 18 F-labeled pyrimidine acrylamide EGFR positron tracer ( 18 F-BF 3 -AZD9291)
[0086] In step 3, 70W microwave is used for heating, the heating time can be shortened by 1-2 minutes, the synthesis efficiency can reach 30%, and the radiochemical purity is greater than 98%. Other steps are the same as in embodiment 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com