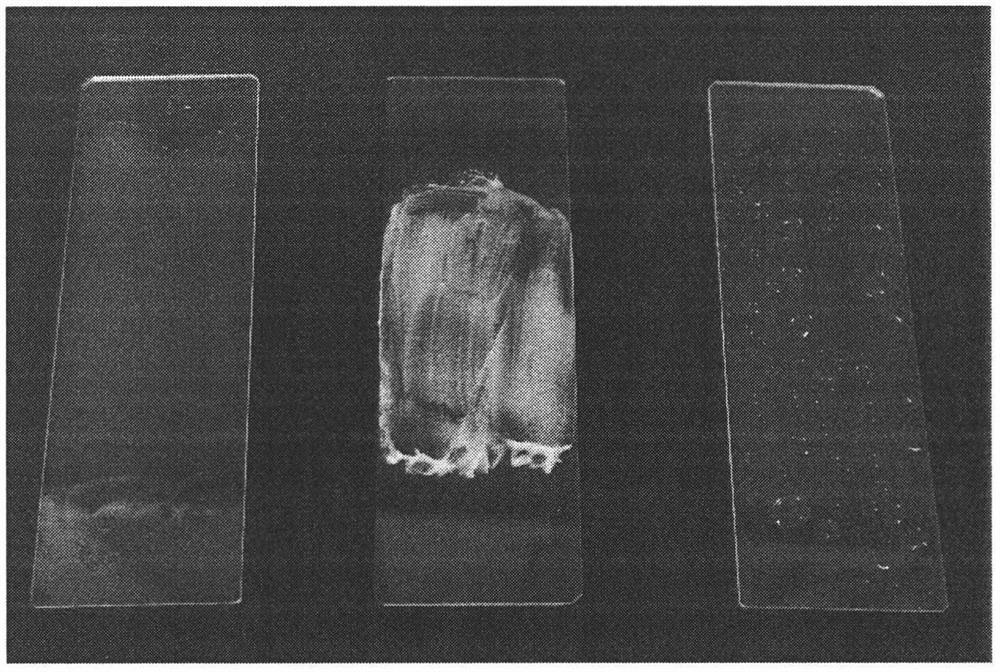
A kind of transdermal delivery system of terbinafine hydrochloride long-acting film spray
A technology of terbinafine hydrochloride and terbinafine, which is applied in the direction of non-active ingredient medical preparations, medical preparations containing active ingredients, skin diseases, etc., and can solve the problem of short residence time, easy to be stained by socks, underwear, etc. And other problems such as friction, to achieve good film-forming performance, good tensile strength, and promote the effect of granulation re-growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
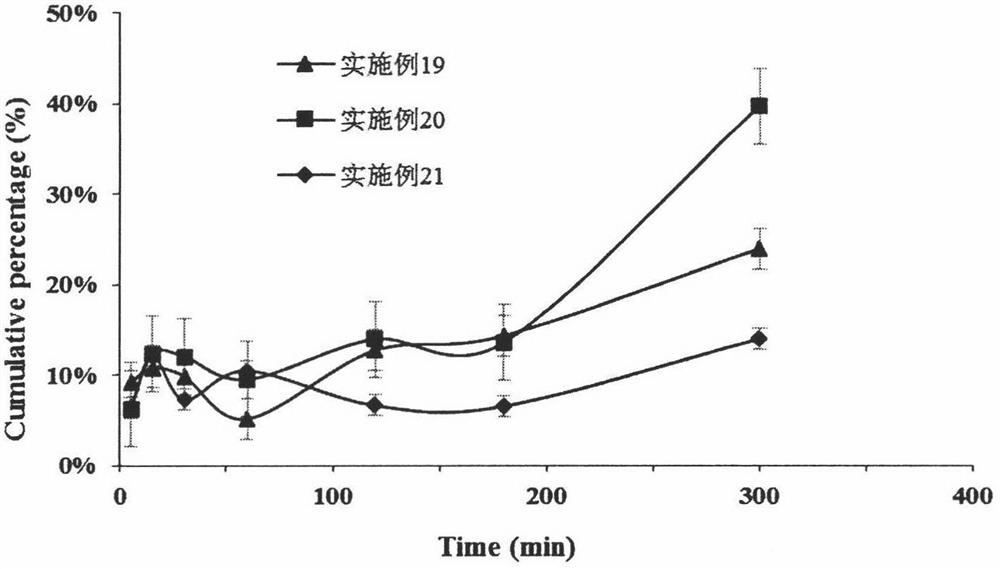
Examples
Embodiment 1
[0068]
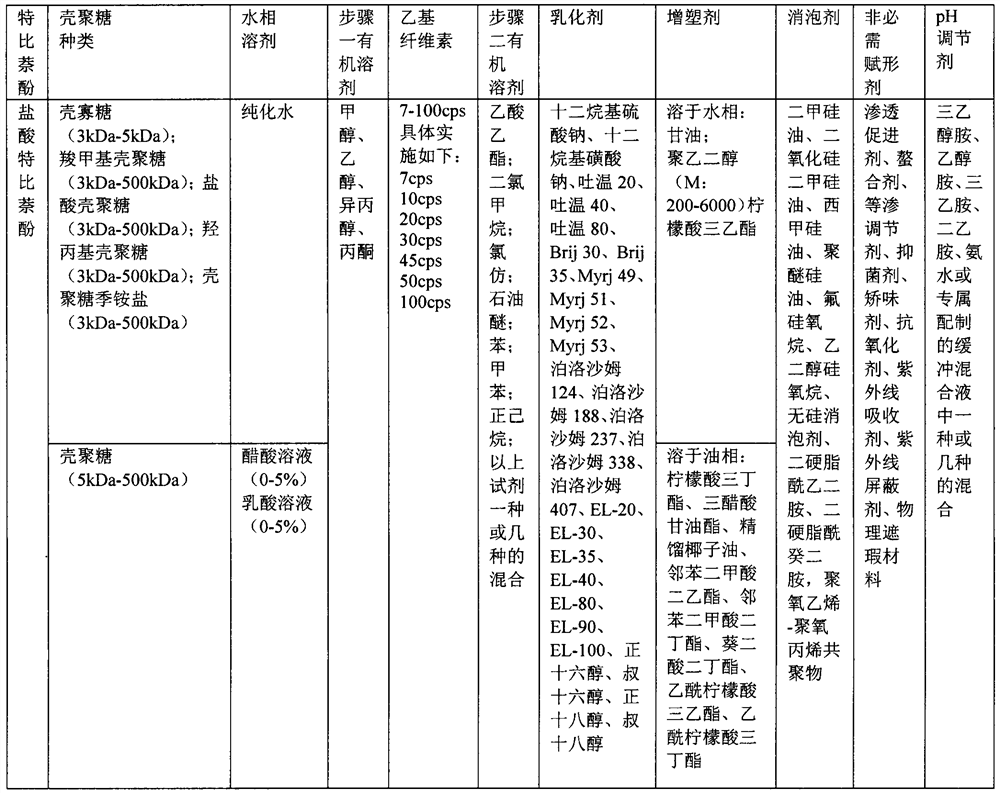
[0069] Step 1: Add 0.1g chitosan oligosaccharide-3kDa into purified water, stir to dissolve, and form chitosan oligosaccharide solution for later use; add 0.02g terbinaol hydrochloride and 0.1g poloxamer 188 to 5mL methanol, stir to dissolve , into a drug-loaded organic solution for later use. Fully mix the chitosan solution and the drug-loaded organic solution, continue to stir for 30 minutes to 60 minutes, remove the organic reagent by rotary evaporation under reduced pressure, then add triethyl citrate, stir and dissolve, which is the "water phase".
[0070] Step 2: Add 0.2 g of ethyl cellulose with a viscosity of 7 cp into 5 mL of ethyl acetate and stir to dissolve, which is the "oil phase".
[0071] Step 3: Fully mix the "water phase" and "oil phase", stir magnetically for 30-60 minutes to form colostrum, 600W probe ultrasonic 10min to form end-milk, remove organic reagents by rotary evaporation under reduced pressure, add 0.02g of simethicone , add water to ...
Embodiment 2
[0073]
[0074] Step 1: Add 0.2 g of terbinaphol hydrochloride and 0.6 g of poloxamer 407 into 10 mL of methanol, stir to dissolve, and form a drug-loaded organic solution for later use. Slowly add 40mL of purified water into it dropwise, continue to stir for 30min-60min, remove the organic reagent by rotary evaporation under reduced pressure, and obtain a drug-loaded aqueous solution, add 0.4g of chitosan oligosaccharide-5kDa, stir and dissolve, which is the "water phase".
[0075] Step 2: Add 0.8 g of ethyl cellulose with a viscosity of 10 cp into 10 mL of chloroform, stir to dissolve, add 0.2 g of tributyl citrate, stir to dissolve each other, which is the "oil phase".
[0076] Step 3: Fully mix the "water phase" and "oil phase", stir magnetically for 40-80 minutes to form colostrum, 300W probe ultrasonic for 15 minutes to form end-milk, remove organic reagents by rotary evaporation under reduced pressure, and add 0.12g of silicon dioxide Simethicone, add water to make u...
Embodiment 3
[0078]
[0079] Step 1: Add 0.24 g of terbinaphol, 0.96 g of poloxamer 188 (F68) and sodium dodecyl sulfate (SDS) into 8 mL of ethanol, stir and dissolve to form a drug-loaded organic solution, and set aside. Slowly add 30mL of purified water dropwise, continue to stir for 30min~80min, remove the organic reagent by rotary evaporation under reduced pressure, and obtain the drug-loaded aqueous solution, add 0.45g of carboxymethyl chitooligosaccharide-9kDa, stir and dissolve, which is the "water phase" .
[0080] Step 2: Add 0.3 g of ethyl cellulose with a viscosity of 20 cps into 7 mL of petroleum ether, stir to dissolve, add 0.15 g of glycerol triacetate, stir to dissolve each other, which is the "oil phase".
[0081] Step 3: Fully mix the "water phase" and "oil phase", stir magnetically for 50-90 minutes to form colostrum, 400W probe to sonicate for 15 minutes to form end-milk, remove organic reagents by rotary evaporation under reduced pressure, add 0.12g of simethicone, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com