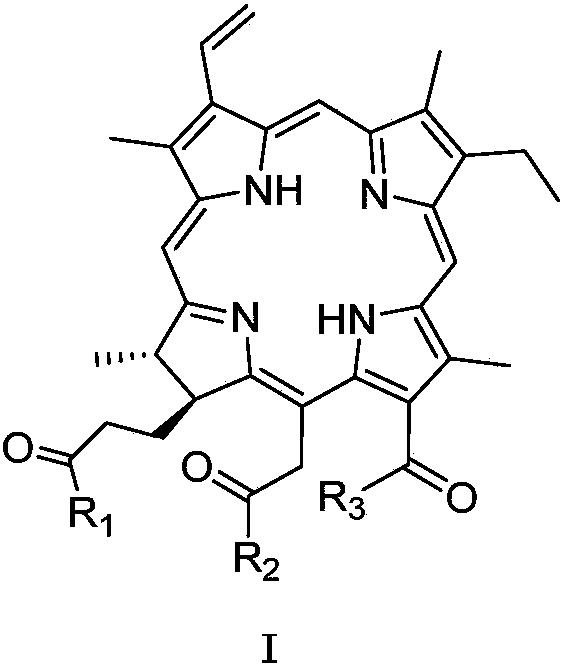
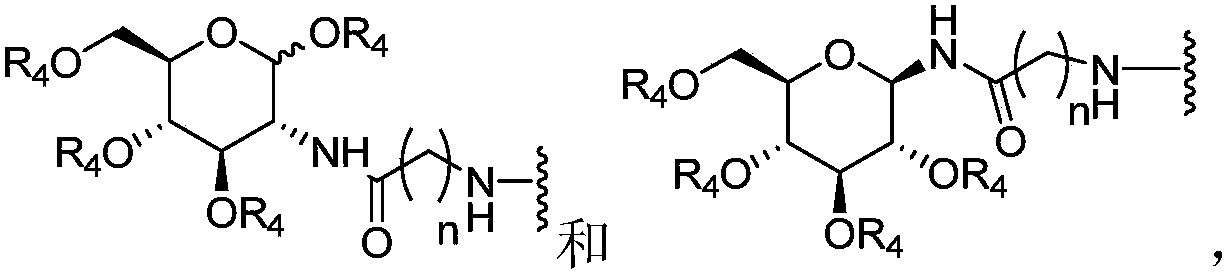
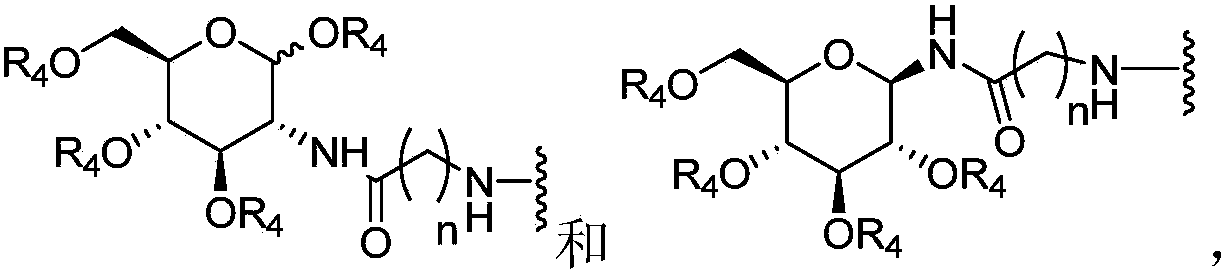
Chlorins glucoside compound, and preparation method and application thereof
A technology of glucoside and chlorin, which is applied in the field of chemical medicine, can solve the problems of complex method, small absorption coefficient, poor tumor cell selectivity, etc., and achieves the effects of simple synthesis method, easy control of conditions and favorable production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Synthesis of compound 3b
[0049] Compound 1 (CHC) (100.0 mg) was dissolved in DMF (1.7 ml). N 2 Protected and stirred in an ice bath. EDCI (38.5 mg) was added, stirring continued in the ice bath, and the ice bath was removed. Stir at room temperature for 2.5 h, dissolve 1,3,4,6-tetra-O-acetyl-beta-D-glucosamine hydrochloride (83.7 mg) in DMF (1 ml), add 40 μl of triethylamine, Mix well. Add it into the reaction solution, stir at room temperature, and monitor the termination of the reaction by TLC. The reaction solution was diluted with 50 ml of dichloromethane, and extracted with aqueous citric acid (25 mL×3). The organic phase was dried, filtered and the filtrate was concentrated. Separation by column chromatography, the elution condition was (dichloromethane / methanol=9:1), and intermediate product 2b (58.1 mg) was obtained;
[0050] Dissolve compound 2b in DMF (0.6ml), add iodomethane (31μl), potassium carbonate (150mg), and react the mixed solution at room te...
Embodiment 2
[0055] Synthesis of compound 6b
[0056] Dissolve methylated chlorophyllin a (100.0 mg) in CHCl 3 (1.8ml), N 2 Protected and stirred in an ice bath. EDCI (38.5 mg) was added, stirring continued in the ice bath, and the ice bath was removed. Stir at room temperature for 2.5 h, dissolve 1,3,4,6-tetra-O-acetyl-beta-D-glucosamine hydrochloride (83.7 mg) in DMF (1 ml), add 40 μl of triethylamine, Mix well. Add it into the reaction solution, stir at room temperature, and monitor the termination of the reaction by TLC. The reaction solution was diluted with 50 ml of dichloromethane, and extracted with aqueous citric acid (25 mL×3). The organic phase was dried, filtered and the filtrate was concentrated. Separation by column chromatography, the elution condition is (dichloromethane / methanol=9:1), to obtain the intermediate product 5b (55.1mg); the compound 5b was dissolved in methanol (0.6ml), and 1 times the amount (molar ratio) of methanol was added Sodium, the mixed solution...
Embodiment 3
[0061] Synthesis of compound 11
[0062]
[0063] Compound 1 (100 mg) was dissolved in 5% H 2 SO4 / MeOH solution (2ml), N 2 Protected, stirred at room temperature, and monitored by TLC to terminate the reaction. Rotary evaporator concentrated to remove methanol. Dilute the reaction solution with dichloromethane (25ml), wash with deionized water, aqueous sodium bicarbonate solution, and saturated NaCl water once each (25ml). The organic phase was dried, filtered and the filtrate was concentrated. After separation by column chromatography, the elution condition was (dichloromethane / methanol=19:1), and 97 mg was obtained, with a yield of 97%. Compound 8: 1 H NMR (400MHz, CDCl3) δ9.64 (1H, s, 10-H), 9.50 (1H, s, 5-H), 8.72 (1H, s, 20-H), 8.01 (1H, dd, J= 17.6, 11.6Hz, 3 1 -H),6.32(1H,dd,J=17.6Hz,1.2Hz,3 2a -H),6.12(1H,dd,J=11.6,1.2Hz,3 2b -H),5.51(1H,d,J=18Hz,15 1a -H),5.26(1H,d,J=18Hz,15 1b -H),4.46(1H,q,J=7.6Hz,18-H),4.12(1H,d,J=7.2Hz,17-H),3.82(3H,s,12 1 -H),3.74(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com