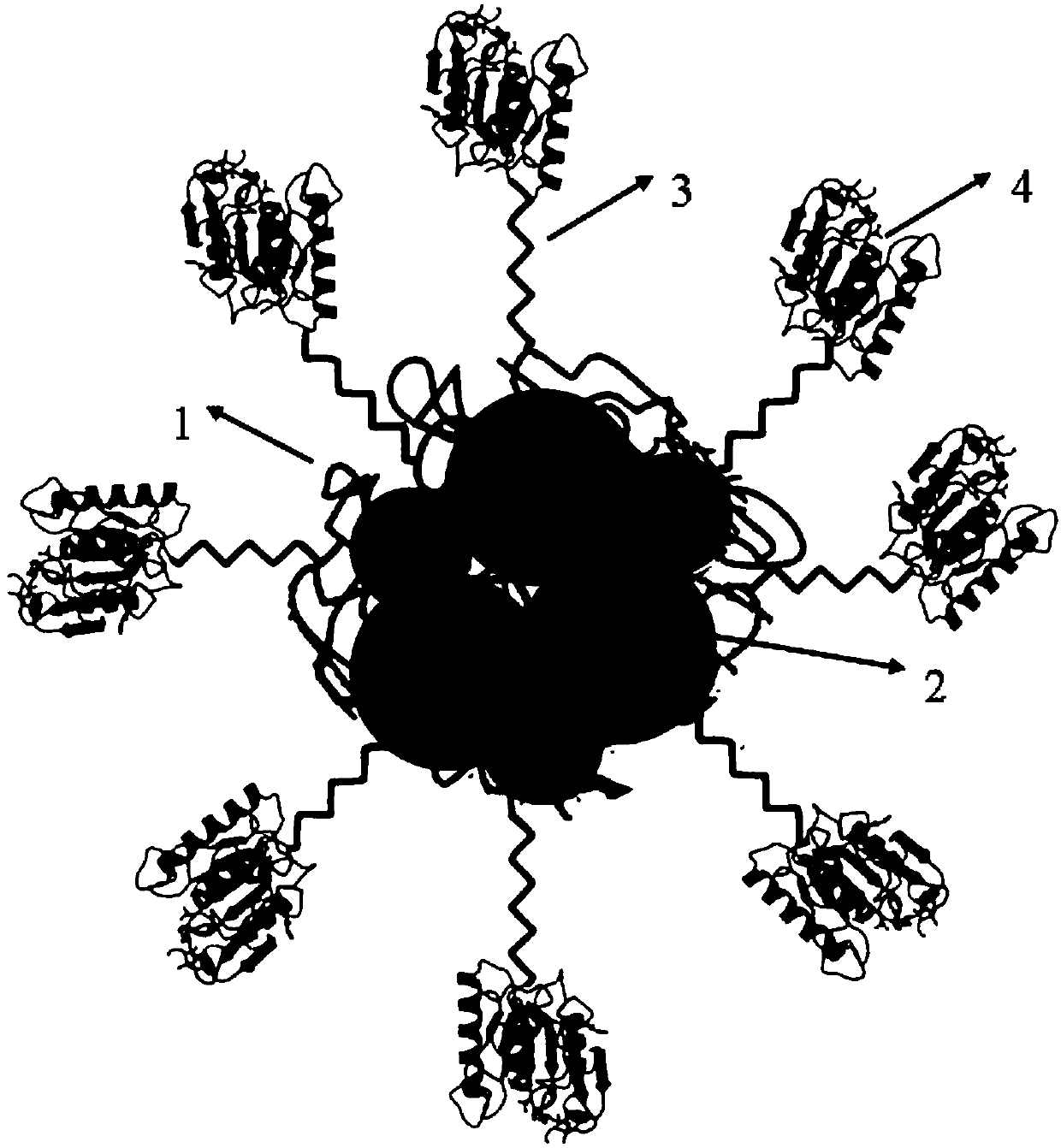
Zwitter-ion magnetic composite hydrogel immobilized enzyme supporter and preparation method
An immobilized enzyme carrier, composite hydrogel technology, applied in biochemical equipment and methods, immobilized on or in an inorganic carrier, immobilized on/in an organic carrier, etc., can solve the biological phase of polymer materials. The problems of low capacity, large influence of enzyme activity, and difficult separation are achieved, achieving the effects of good stability and reusability, high recovery rate of enzyme activity, and good mechanical properties.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
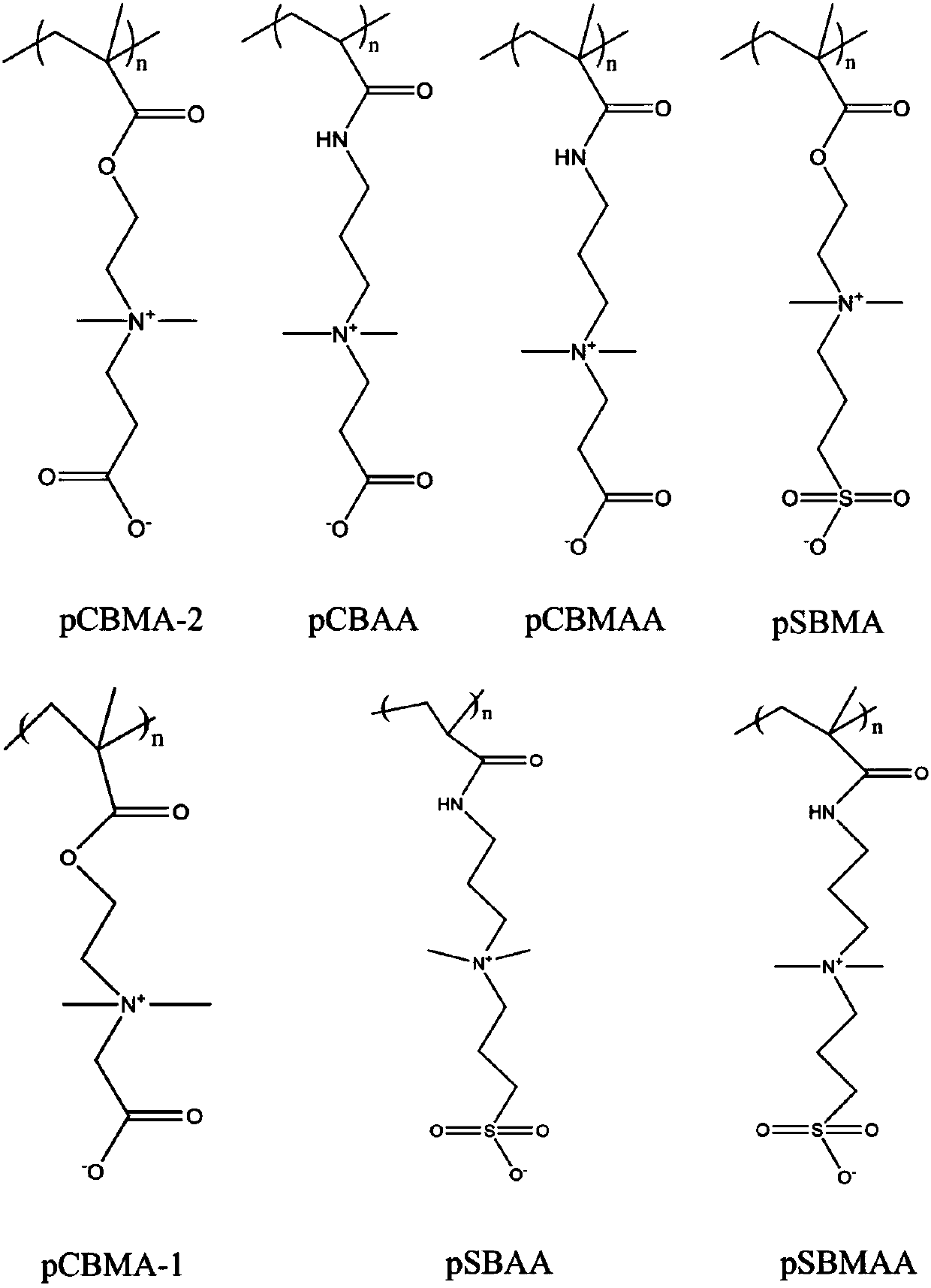
[0048] Preparation of carboxybetaine acrylamide (CBAA) zwitterionic magnetic hydrogel carrier by template method
[0049] (1) Add 300μl of ultrapure water to a 1.5ml centrifuge tube, weigh 0.1g of ferric oxide 2 and place it in a centrifuge tube, and add a compound surfactant: 20μl Span+20μl Tween, in Vibrate on a vortex shaker for several seconds to disperse the ferric oxide 2 to prepare a stable compound surfactant-dispersed ferric oxide dispersion system.
[0050] (2) Weigh 0.006g N,N'-methylenebisacrylamide (MBAA), 0.6g carboxybetaine acrylamide (CBAA) monomer, 0.0016g ammonium persulfate (APS), 2μl tetramethylethyl ether Diamine (TEMED) is dissolved in 1ml of distilled water, and the mass percentages of the above components are as follows:
[0051] Carboxybetaine acrylamide (CBAA) monomer 37.35%
[0052] N,N'-methylenebisacrylamide (crosslinking agent) 0.37%
[0053] Ammonium persulfate-tetramethylethylenediamine (initiator) 0.02%
[0054] Distilled water (solvent) 62...
Embodiment 2
[0060] Preparation of carboxybetaine methacrylamide (CBMAA) zwitterionic magnetic hydrogel carrier by template method
[0061] (1) Add 600 μl of ultrapure water to a 1.5ml centrifuge tube, weigh 0.2 g of ferric oxide 2 and place it in a centrifuge tube, and add 0.05 g of gum arabic to it, shake it on a vortex shaker for a few seconds, Disperse iron ferric oxide 2 to prepare a stable gum arabic dispersed iron ferric oxide dispersion system.
[0062] (2) Weigh 0.041g N,N'-dimethylacrylamide cystine, 0.194g carboxybetaine methacrylamide (CBMAA) monomer, 0.005g azobisisoheptanonitrile, dissolve in 1.7ml distilled water In, the mass percent of above-mentioned components is as follows:
[0063] Carboxybetaine methacrylamide (CBMAA) monomer 10.00%
[0064] N,N'-Dimethacrylamide cystine (cross-linking agent) 2.09%
[0065] Azobisisoheptanonitrile (initiator) 0.26%
[0066] Distilled water (solvent) 87.65%
[0067] Add 600 μl of ferric oxide dispersion to the above solution. Put ...
Embodiment 3
[0072] Preparation of carboxybetaine methacrylate-1 (CBMA-1) zwitterionic magnetic hydrogel carrier by template method
[0073] (1) Add 300μl of ultrapure water to a 1.5ml centrifuge tube, weigh 0.1g of ferric oxide 2 and place it in a centrifuge tube, add 0.039g of sodium citrate to it, and shake it on a vortex shaker for a few seconds , so that ferric oxide 2 is dispersed to make a stable sodium citrate dispersed iron ferric oxide dispersion system.
[0074] (2) Take 4.29μL polyethylene glycol diglycidyl ether, 0.692g carboxybetaine methacrylate-1 (CBMA-1) monomer, 0.001g ammonium persulfate (APS), 2μl tetramethylethylene glycol The amine (TEMED) was dissolved in 1 ml of 1M sodium chloride solution. The mass percentages of the above-mentioned components are as follows:
[0075]
[0076] Add 300 μl of ferric oxide dispersion to the above solution. Put the gel-forming precursor mixed solution on a vortex shaker and mix evenly.
[0077] Carboxybetaine methacrylate-1 (CBM...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com