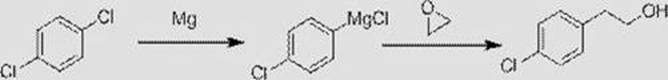
A kind of synthetic method of p-chlorophenyl alcohol
A technology of chlorophenethanol and synthesis method, which is applied in the direction of chemical instruments and methods, magnesium organic compound, hydroxyl compound preparation, etc., can solve the problems that are not conducive to safety and environmental protection, difficult to separate and purify, and prone to violent heat release, and achieve comprehensive raw materials Low cost, easy separation and purification, and high safety factor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
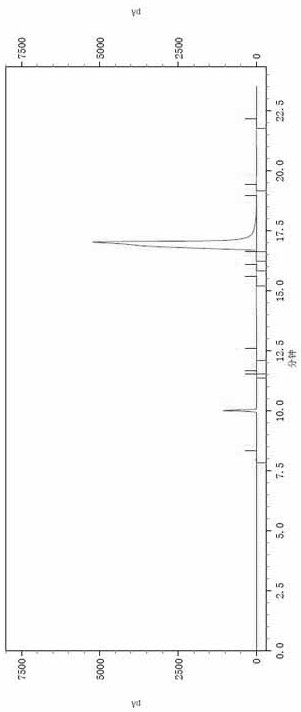
Image
Examples
Embodiment 1
[0031] A kind of synthetic method of p-chlorophenethyl alcohol of the present embodiment, comprises the following steps:
[0032] (1) Add 800g of 2-methyltetrahydrofuran and 29.4g of magnesium chips to a four-necked round-bottom reaction flask equipped with a mechanical stirrer, a reflux condenser and a thermometer. The mass fraction of water in the 2-methyltetrahydrofuran is 0.01 %, use nitrogen to replace the air in the reaction flask, stir to control the temperature in the reaction flask to 60-65°C, add bromoethane 1.5g, the reaction solution in the reaction flask will change from light yellow to light gray, continue to stir, control The temperature of the reaction material is 75-80°C. Use the dropping funnel to add 147g of p-dichlorobenzene dropwise. The dropping time of p-dichlorobenzene is controlled at 2.5-3 hours. After 5 hours, reaction solution A was obtained, which contained p-chlorophenylmagnesium chloride Grignard reagent;
[0033] (2) Lower the temperature of th...
Embodiment 2
[0036] A kind of synthetic method of p-chlorophenethyl alcohol of the present embodiment, comprises the following steps:
[0037] (1) Add 200g of 2-methyltetrahydrofuran and 28.8g of magnesium chips to a four-necked round-bottom reaction flask equipped with a mechanical stirrer, a reflux condenser and a thermometer. The mass fraction of water in the 2-methyltetrahydrofuran is 0.02 %, use nitrogen to replace the air in the reaction flask, stir to control the temperature of the material to 60°C, add 5.5g of elemental iodine, the reaction solution in the reaction flask will change from light brown to light gray, continue stirring, heating, and control the temperature in the reaction flask 85-90°C, use the dropping funnel to add 147g of p-dichlorobenzene drop by drop, the dropping time of p-dichlorobenzene is controlled at 1.5-2 hours, after the addition, keep warm at 85-90°C and stir for 2-3 hours. The reaction solution A is obtained, and the reaction solution A contains p-chloroph...
Embodiment 3
[0041] A kind of synthetic method of p-chlorophenethyl alcohol of the present embodiment, comprises the following steps:
[0042] (1) Add 600g of tetrahydrofuran and 28.8g of magnesium chips into a four-necked round-bottomed reaction flask equipped with a mechanical stirrer, reflux condenser and thermometer. The mass fraction of water in the tetrahydrofuran is 0.01%, and the reaction flask is replaced with nitrogen. Stir and control the temperature in the reaction flask to be 50°C, add 1.5g of ethyl bromide dropwise, the reaction solution in the reaction flask will change from light yellow to light gray, continue stirring and heating, and control the temperature of the material to 70-75°C 147g of p-dichlorobenzene was added dropwise using a dropping funnel, and the dropping time of p-dichlorobenzene was controlled to be 1.5-2 hours. Contain p-chlorophenylmagnesium chloride Grignard reagent in the said reaction solution A;
[0043] (2) Lower the temperature of the above reacti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com