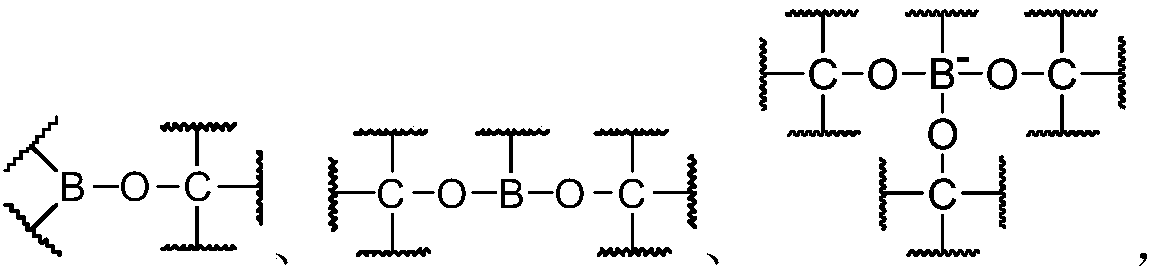
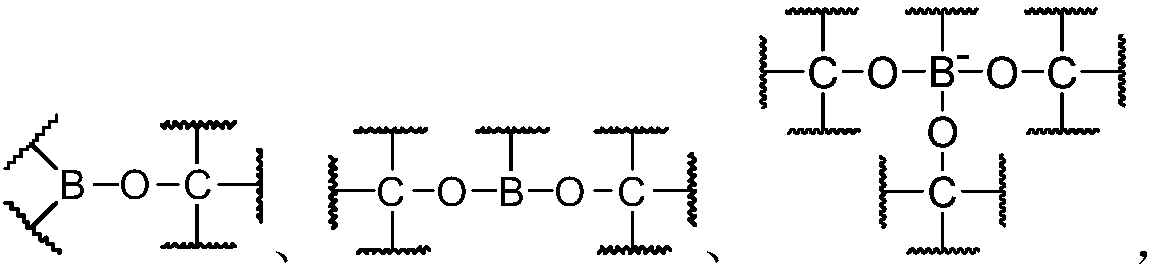
Dynamic polymer and application thereof
A polymer and dynamic technology, applied in the field of intelligent polymers, can solve problems such as poor stability of mercaptans, application limitations, and poor dynamic activity of ester bonds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0401] In the preparation process of dynamic polymer, three methods of mechanical foaming method, physical foaming method and chemical foaming method are mainly used to foam dynamic polymer.
[0402] Among them, the mechanical foaming method is to introduce a large amount of air or other gases into the emulsion, suspension or solution of the polymer with the help of strong stirring during the preparation of the dynamic polymer to make it a uniform foam, and then through physical Or chemical changes make it gel and solidify to become a foam material. In order to shorten the molding cycle, air can be introduced and emulsifiers or surfactants can be added.
[0403] Wherein, the physical foaming method is to utilize physical principles to realize the foaming of the polymer in the preparation process of the dynamic polymer, which generally includes the following four methods: (1) inert gas foaming method, that is, after adding Press the inert gas into the molten polymer or pasty m...
Embodiment 1
[0460] Take a certain amount of binary organic boric acid compound 1 (use ethyl lithium, vinyl lithium and trimethyl borate to react to make ethyl vinyl boronic acid; take AIBN as initiator and triethylamine as catalyst, use ethyl vinyl boronic acid and 1,6-hexanedithiol by thiol-ene click reaction) dissolved in tetrahydrofuran solvent to prepare a 0.5mol / L solution; weigh a certain amount of terminal hydroxypropyl polydimethylsiloxane Diol was dissolved in tetrahydrofuran solvent to prepare a 0.1mol / L solution. Take 10mL tetrahydrofuran solution dissolved with binary organic boronic acid compound 1 and add it to a dry and clean reaction bottle, add a small amount of acetic acid dropwise, stir and mix for 15min, then slowly add 50mL dissolved hydroxypropyl polydimethyl The tetrahydrofuran solution of siloxane diol and 2 mL of triethylamine were stirred and mixed for 10 min. The above mixed solution was continuously stirred at 80°C, and the water produced by the reaction was c...
Embodiment 2
[0462] Firstly, ethyl boron dichloride is hydrolyzed into ethyl boric acid under acidic conditions, and then under the catalysis of triethylamine, organic boric acid and alcohol are synthesized through the esterification reaction of ethyl boric acid and 1,4-butanediol through organic boric acid and alcohol. For the glycol 1 of the borate bond, the molar ratio of ethyl boron dichloride and 1,4-butanediol is controlled to be 1:2; the obtained glycol 1 containing the organic borate group and Under the catalysis of dibutyltin dilaurate, excessive hexamethylene diisocyanate was copolymerized at 85°C to obtain polyurethane-based diisocyanate 2 containing organic borate bonds; then propynyl alcohol and polyurethane containing organic borate bonds Diisocyanate 2 was reacted at a mass ratio of 2:1 to obtain bispropynyl-terminated polyurethane-based diyne compound 3 containing organic boronic ester bonds. The polyurethane-based diyne compound 3 and 1,11-diazido-3,6,9-trioxaundecane cont...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Elastic modulus | aaaaa | aaaaa |
| Elastic modulus | aaaaa | aaaaa |
| Elastic modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com