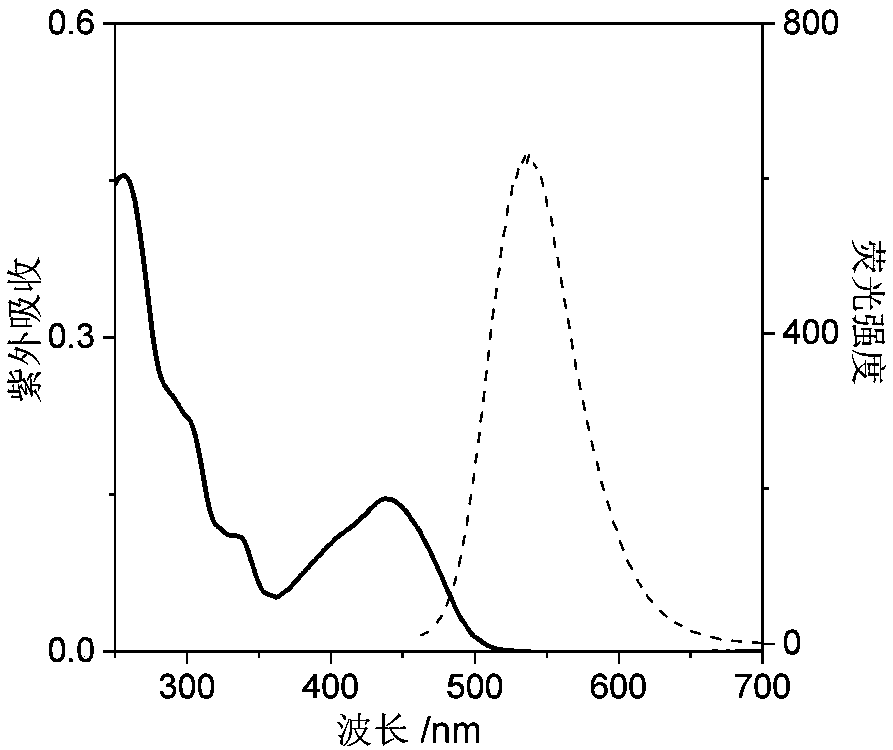
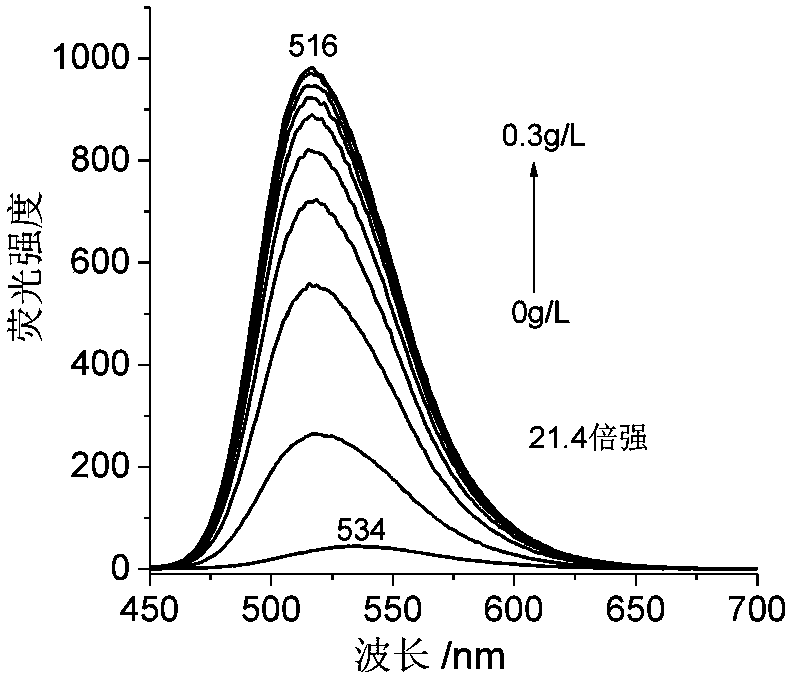
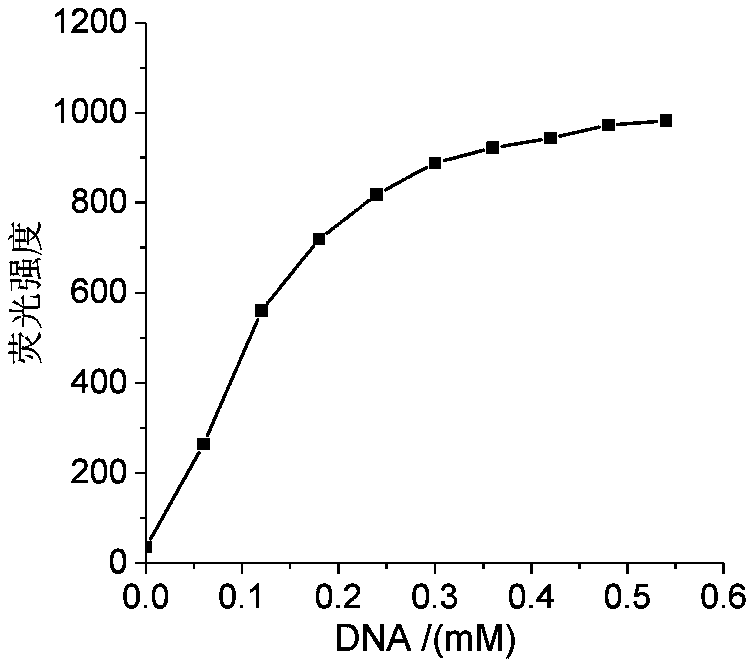
Nucleic acid fluorescence probe for nuclear staining and preparation method of nucleic acid fluorescence probe
A fluorescent probe and cell nucleus technology, applied in the field of cell nucleus fluorescent probes, can solve the problems of low anti-biological metabolism and high biological toxicity, and achieve the effect of strong anti-enzymolysis ability, low cytotoxicity and high nuclear targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A, the preparation of intermediate 1
[0030] At room temperature, dissolve 1.26mmol 6-amino-2-methylquinoline in acetonitrile and add it to a 250ml flask, then slowly add 1.33mmol N-bromosuccinimide and a catalytic amount of silica gel, and continue stirring for 15min Afterwards, the liquid in the bottle turned dark brown, and thin-layer chromatography determined that new spots were generated in the reaction, and the raw materials were not completely reacted, and the reaction was continued for 12 hours. After the reaction was finished, extracted with ethyl acetate, spin-dried the solvent, and the residue was separated and purified through a chromatographic column (ethyl acetate:petroleum ether volume ratio=1:6) to obtain 207 mg of a yellow solid product, namely intermediate 1, with a yield of 69%.
[0031] 1 H NMR (400MHz, CDCl3, TMS): δ = 8.23 (d, J = 8.6Hz, quinoline-H, 1H), 7.81 (d, J = 9.0Hz, quinoline-H, 1H), 7.28 (d, J = 8.6Hz, quinoline-H, 1H), 7.20 (d, J=9...
Embodiment 2
[0045] A. Preparation of Intermediate 1
[0046] At room temperature, 1.26mmol of 6-amino-2-methylquinoline was dissolved in acetonitrile and added to a 250ml flask, then 1.33mmol of N-bromosuccinimide and a catalytic amount of silica gel were slowly added, and continued stirring for 15min After the liquid in the bottle turned dark brown, thin layer chromatography determined that a new point was formed in the reaction, and the raw material was not completely reacted, and the reaction was continued for 16 hours. After the reaction was completed, ethyl acetate was extracted, the solvent was spin-dried, and the residue was separated and purified by a chromatographic column (ethyl acetate: petroleum ether volume ratio = 1:6) to obtain 222 mg of a yellow solid product, namely Intermediate 1, with a yield of 222 mg. was 74%.
[0047] B, the preparation of intermediate 2
[0048] Add 90 mg of intermediate 1 to the round-bottomed flask, dissolve it with 16 ml of a mixed solution of ...
Embodiment 3
[0052] A. Preparation of Intermediate 1
[0053] At room temperature, 1.26mmol of 6-amino-2-methylquinoline was dissolved in acetonitrile and added to a 250ml flask, then 1.33mmol of N-bromosuccinimide and a catalytic amount of silica gel were slowly added, and continued stirring for 15min Afterwards, the liquid in the bottle turned dark brown, and thin-layer chromatography determined that a new point was formed in the reaction, and the raw material was not completely reacted, and the reaction was continued for 18 hours. After the reaction was completed, ethyl acetate was extracted, the solvent was spin-dried, and the residue was separated and purified by a chromatographic column (ethyl acetate: petroleum ether volume ratio = 1:6) to obtain 216 mg of the product in the form of a yellow solid, namely intermediate 1, and the yield was was 72%.
[0054] B, the preparation of intermediate 2
[0055] Add 90 mg of intermediate 1 to the round-bottomed flask, dissolve it with 16 ml ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com