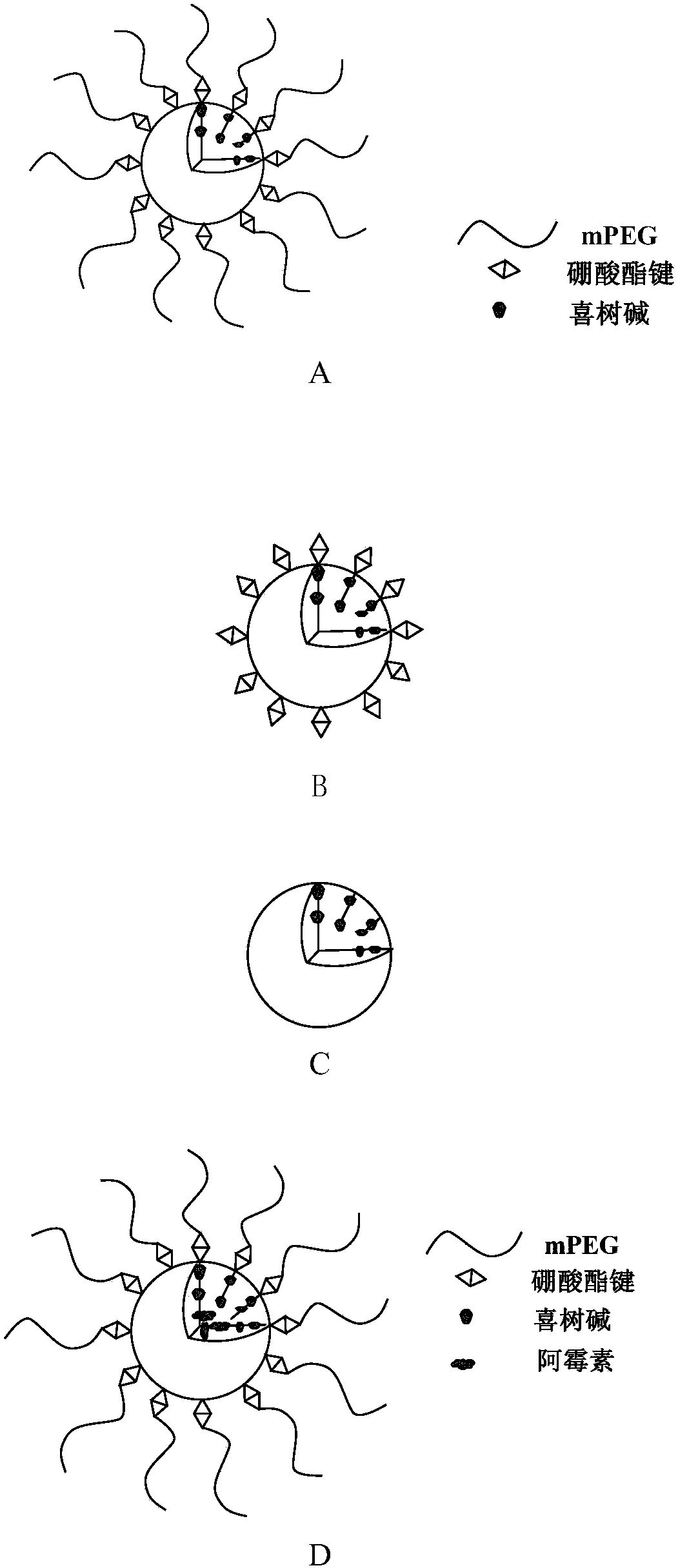
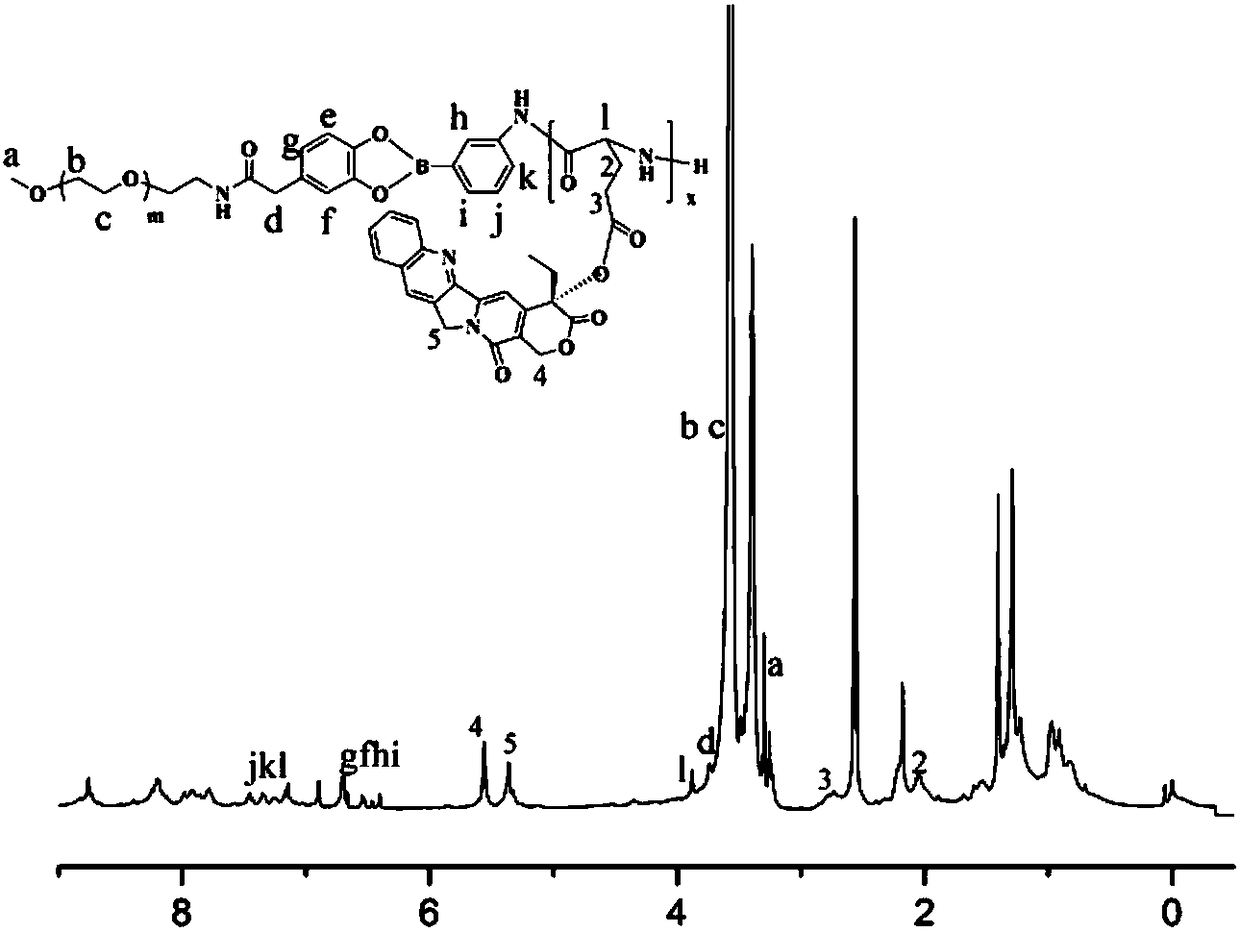
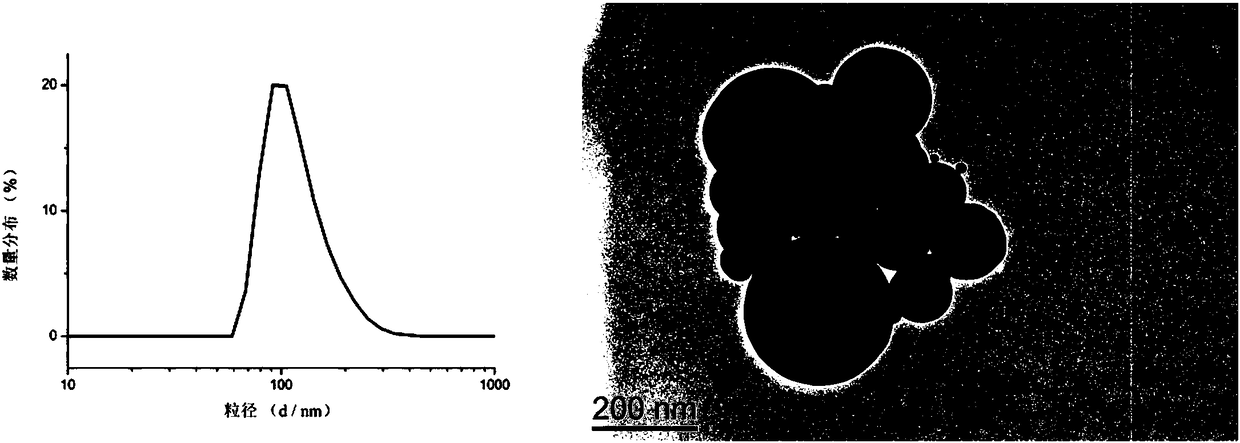
Amphiphilic camptothecin polymer prodrug taking phenylboronic acid ester as connecting unit, as well as preparation method and application thereof
A linking unit and phenylboronic acid ester technology, which is applied in the field of biomedical technology, nanomedicine and new materials, can solve the problems of lack of targeting, no function of fixed-point release of drugs, and reduced activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0119] Embodiment 1, the synthesis of mPEG-3,4-DA
[0120] Weigh 3,4-dihydroxyphenylacetic acid (0.126g, 0.750mmol) and dissolve it in 25mL of anhydrous dichloromethane, disperse it by ultrasonic to form a suspension, add 1-ethyl-(3-dimethylamino Propyl)carbodiimide hydrochloride (0.154g, 0.800mmol), 1-hydroxybenzotriazole (0.108g, 0.800mmol), N,N-diisopropylethylamine (0.260mL, 1.500mmol ), after 0.5h, add mPEG-NH under nitrogen protection 2 (1.000g, 0.500mmol), the reaction device was protected from light, reacted overnight at room temperature, concentrated, added pure water to dissolve, extracted several times with dichloromethane, dried the organic phase with anhydrous sodium sulfate, concentrated the filtrate and mixed the sample and purified it through a silica gel column. The mobile phase was mixed with dichloromethane and methanol in a certain volume ratio (30:1). After the purified product was concentrated, it was added to glacial ether to precipitate, and the white...
Embodiment 2
[0124] The synthesis of embodiment 2, mPEG-BC
[0125]Weigh 3-aminophenylboronic acid (0.191g, 1.390mmol) and 200mL redistilled toluene into a 500mL single-necked flask, ultrasonically promote its dissolution, add mPEG-3,4-DA (0.300g, 0.139mmol) under nitrogen protection , react at 120°C for 5 hours, concentrate the reaction solution, add 30 mL of anhydrous tetrahydrofuran to dissolve, transfer the tetrahydrofuran solution to a dialysis bag with a molecular weight cut-off of 1000, dialyze the anhydrous tetrahydrofuran for 5 hours, add it dropwise to 100 mL of ice ether, wash and filter After three times, 0.360 g of light yellow powder mPEG-BC was obtained with a yield of 87%.
[0126] The structural formula of the mPEG-BC is shown in formula (2);
[0127]
[0128] Among them, m=44.
Embodiment 3
[0129] Embodiment 3, the synthesis of Boc-Glu(CPT)-OtBu
[0130] Weigh Boc-L-glutamate-1-tert-butyl ester (1.7416g, 5.74mmol), 4-dimethylaminopyridine (0.1754g, 1.44mmol), 1-hydroxybenzotriazole (0.4654g, 3.44 mmol), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (0.6604g, 3.44mmol), triethylamine (0.48mL, 3.44mmol), and 110mL of anhydrous di Chloromethane was placed in the reaction flask. After 0.5h, camptothecin (1.000g, 2.87mmol) was added under nitrogen protection. After reacting at room temperature for 5h, the reaction solution became clear. Add the aqueous hydrochloric acid solution of pH 1 to terminate the reaction, extract three times with saturated NaCl aqueous solution, anhydrous NaSO Dry the organic phase, after filtering, the filtrate is concentrated and mixed and purified by a silica gel column, and the mobile phase is dichloromethane and methanol by a certain volume ratio (300: 1) Mixing, the purified product is 1.54g of light yellow powder Boc-Glu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com