A kind of temsirolimus liposome and preparation method thereof
一种西罗莫司脂、替西罗莫司的技术,应用在医药领域,能够解决成药可能性低、操作繁琐、大刺激性等问题,达到增加化学稳定性、制备工艺简单、临床使用方便的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1 Study on the druggability of temsirolimus with different formulations
[0059] The drug-forming properties of sulfobutyl ether-β-cyclodextrin inclusion complex, liposome and fat emulsion were respectively studied, and the fixed drug loading amount was 1 mg / ml for parallel comparison. The main research plans and results are summarized as follows:
[0060] 1. Sulfobutyl ether-β-cyclodextrin inclusion complex
[0061] 1.1 Prescription process one
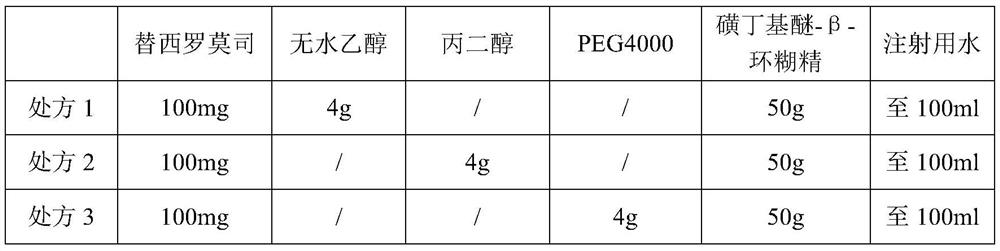
[0062] 1.1.1 Prescription
[0063] temsirolimus Sulfobutyl ether-β-cyclodextrin Water for Injection prescription 1 100mg 10g to 100ml prescription 2 100mg 30g to 100ml prescription 3 100mg 50g to 100ml
[0064] 1.1.2 Preparation method
[0065] Weigh the recipe amount of sulfobutyl ether-β-cyclodextrin, add appropriate amount of water for injection to dissolve, and make up to 100ml; Weigh the recipe amount of temsirolimus, add it to the aqueous solution of sulfobutyl...
Embodiment 2
[0088] The key of embodiment 2 PEGylated phospholipids to the development of temsirolimus liposomes
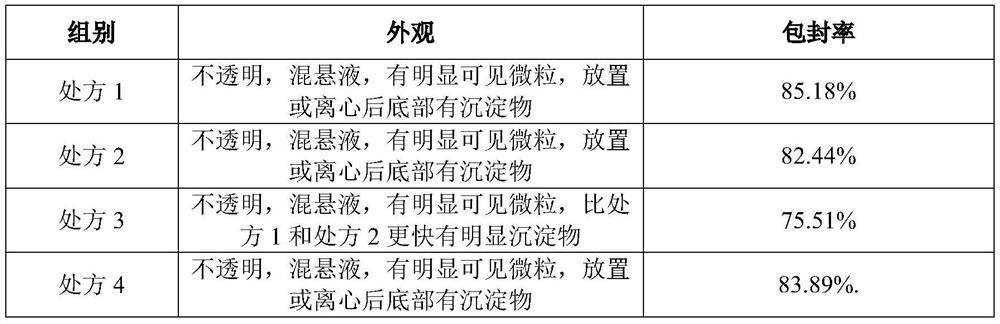
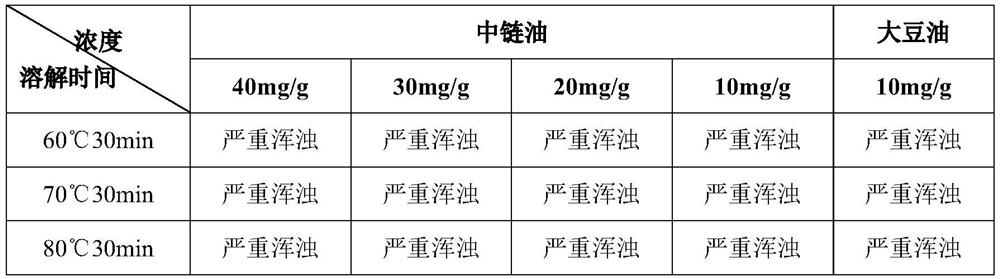
[0089] Usually liposomes are composed of lecithin or lecithin and cholesterol. However, for temsirolimus, an appropriate amount of PEGylated phospholipids must be added to the prescription. Otherwise, no matter how the prescription and process are adjusted, stable liposomes cannot be prepared, and the problems of turbidity and precipitation will occur in a very short period of time. . A typical verification scheme is shown below.
[0090] Take DSPE-PEG2000 as an example:
[0091] 1. Prescription:
[0092] component prescription 1 prescription 2 prescription 3 prescription 4 prescription 5 prescription 6 temsirolimus 125mg 125mg 125mg 125mg 125mg 125mg EPCS 3.5g 4.5g 5.5g 3.5g 3.5g 3.5g cholesterol 0.2g 0.2g 0.2g 0.2g 0.2g 0.2g DSPE-PEG2000 / / / 10mg 50mg 100mg Ethanol 4ml 4ml 4ml 4ml 4ml 4...
Embodiment 3
[0103] Example 3 Preparation of temsirolimus liposomes
[0104] Weigh 0.15 g of temsirolimus, 3.5 g of high-purity egg yolk lecithin (EPCS), 0.125 g of DSPE-PEG2000, and 0.03 g of α-tocopherol, add 8.0 g of tert-butanol, heat to dissolve at 45°C, and place in In the sample dish, the organic solvent was removed by freeze-drying to obtain the lipid phase; 0.01 g of EDTA-2Na and 75 g of water for injection were weighed, heated to 45°C, and dissolved to obtain the aqueous phase; the aqueous phase was added to the lipid phase, and stirred to It is fully dissolved and dispersed to obtain crude liposome; the crude liposome is placed in an extruder, and extruded through extrusion membranes with pore diameters of 0.2 μm, 0.1 μm, and 0.05 μm in turn to obtain a liposome solution; Weigh maltose 20g, placed in the above-mentioned liposome solution, stirred to dissolve, and the volume was adjusted to 100ml with water for injection; the pH value was adjusted to 5.5 with citric acid and sodi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com