HA-targeted layered doubled hydroxide-ultrafine iron nano material and preparation and applications thereof
A hydroxide and nanomaterial technology, which is applied to preparations for in vivo testing, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve the problem of not finding doxorubicin and ultra-small iron nanoparticles. Particle research reports, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Synthesis of materials:
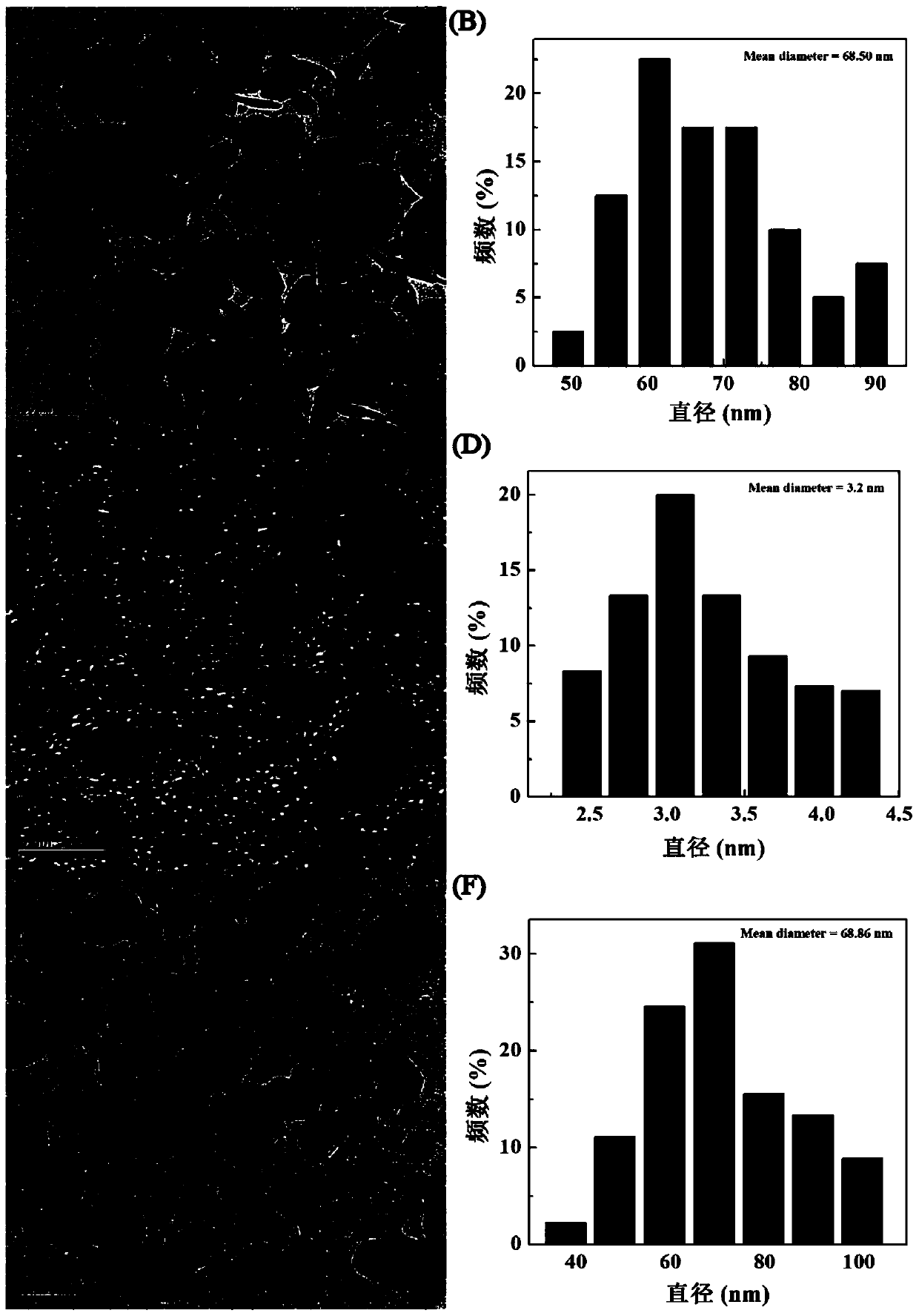
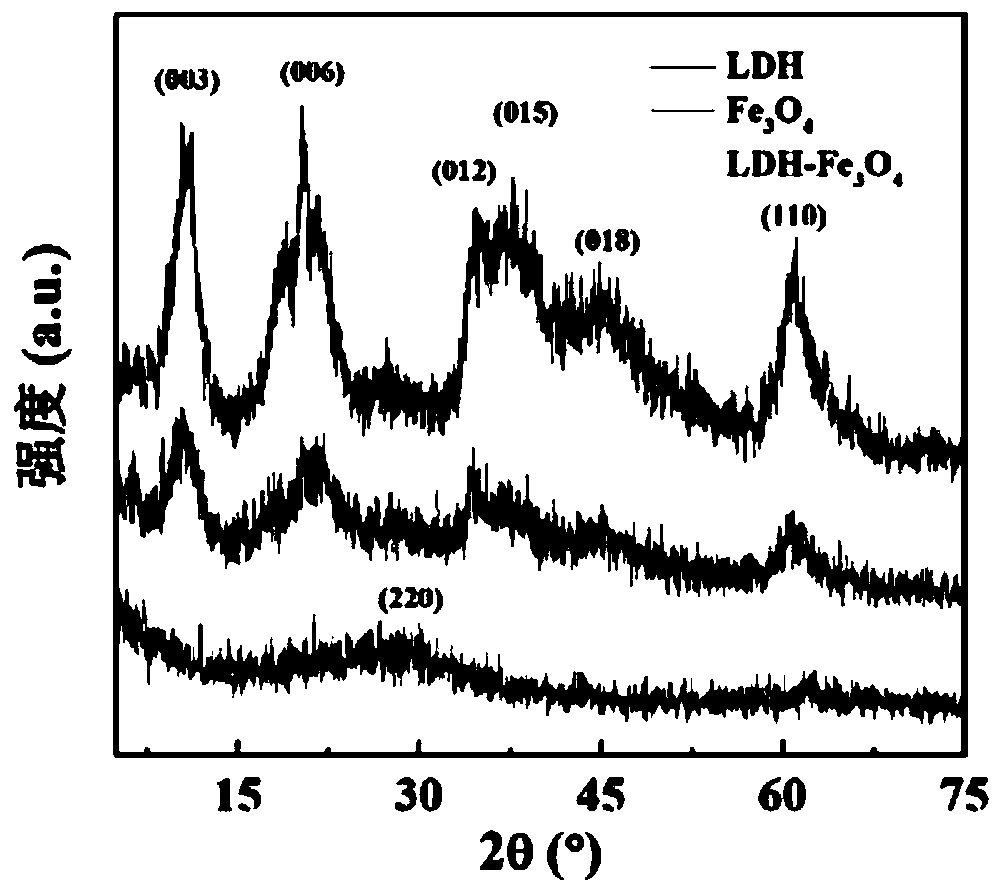
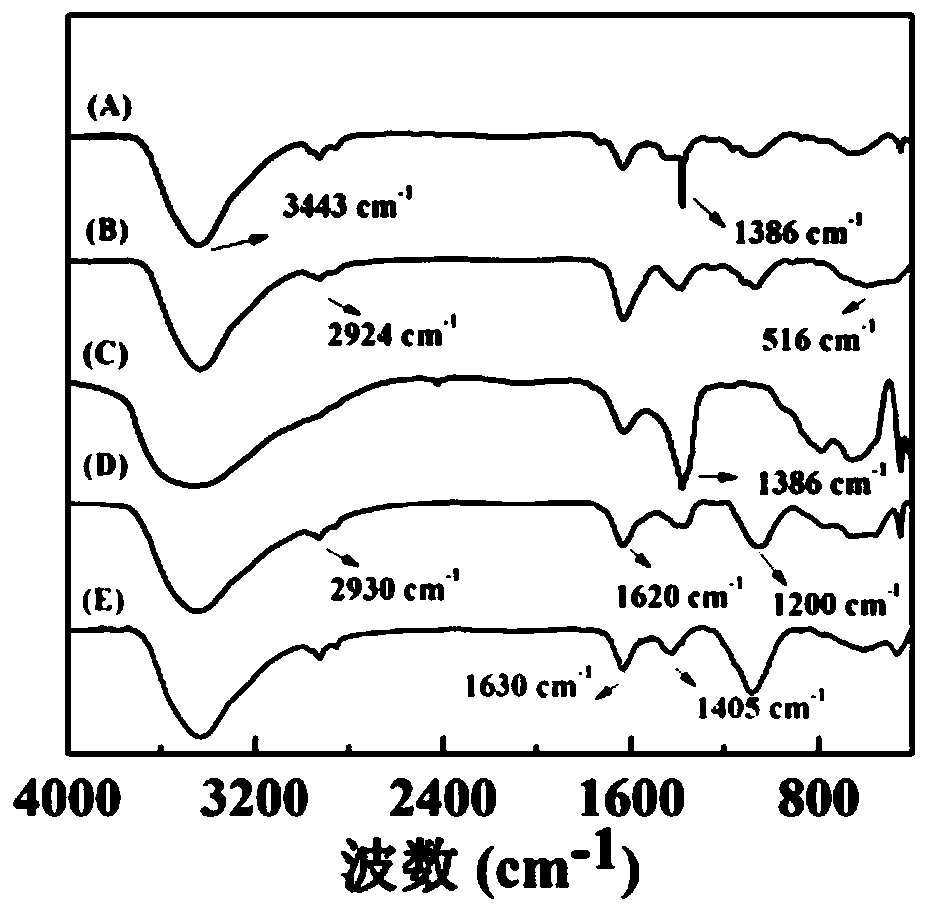
[0081] (1) Mix FeCl under stirring 3 (1081 mg, Adamas reagent, 500 g, batch: P1276861) was dissolved in 40 mL of diethylene glycol to form a homogeneous solution. Sodium citrate (471 mg, Sinopharm Group, 500 g, batch number: 20140611) was added to the above solution, and the mixture was heated to 80° C. in a water bath until a clear solution was formed. Subsequently, sodium acetate (1312 mg, Shanghai Lingfeng Chemical Reagent, 500 g, batch number: 20140124) was added to the above mixture solution and dissolved, and then the mixture was transferred to a Teflon-lined stainless steel autoclave with a volume of 100 mL and sealed in air . The autoclave was placed in an oven at 200 °C for 4 h. After cooling to room temperature, the black solution was collected by centrifugation (10000 rpm, 5 min) and purified 3 times with ethanol to remove excess reactants and by-products. The resulting black product was redispersed into water and lyophilized to ...
Embodiment 2
[0086] To evaluate LDH-Fe 3 o 4 - Imaging effect of HA as an MRI contrast agent, combining the material with pure Fe 3 o 4 r of nanoparticles 1 Relaxation rate for comparison, r 1 The relaxation rate is the longitudinal relaxation time per molar concentration of iron, which can be changed by different concentrations of T 1 The inverse of the relaxation time was calculated by fitting. Measure the Fe prepared in Example 1 by ICP-AES test method 3 o 4 , LDH-Fe 3 o 4 and LDH-Fe 3 o 4 - Content of Fe element in HA. Prepare LDH-Fe with Fe concentration of 0.1, 0.2, 0.4, 0.8 and 1.6mM respectively 3 o 4 -500 μL of HA aqueous solution, the T of the material at different Fe concentrations was measured by a magnetic resonance imaging analyzer 1 relaxation effects (such as Figure 8 ). Calculated Fe 3 o 4 , LDH-Fe 3 o 4 and LDH-Fe 3 o 4 -HA r 1 The values are 0.42, 5.53 and 4.38mM respectively -1 the s -1 . LDH-Fe 3 o 4 -HA r 1 higher than Fe 3 o 4 the r ...
Embodiment 3
[0088] Using B16 cells as a model, using CCK-8 method to detect LDH-Fe 3 o 4 - Cytotoxicity of HA NPs and LDH-Fe 3 o 4 -In vitro anticancer effect of HA / DOX NPs. The concentration of prepared iron is 1000μg / mL LDH-Fe 3 o 4 -The PBS mother solution of HA NPs, and then prepare the concentration of DOX with sterile PBS on the ultra-clean bench and add 10 μL of LDH-Fe of different concentrations 3 o 4- HA / DOX in PBS. The final DOX concentration was 1.6, 3.2, 6.3, 12.5 and 25 μg / mL, and the carrier content corresponding to the highest concentration was used as a group. And sterilized overnight with ultraviolet radiation. Place the cell culture plate in 5% CO 2 , continue to incubate at 37°C for 24h and 48h. Then discard the culture medium, wash it twice with PBS, add 100 μL new culture medium (containing 10 μL CCK-8, 90 μL culture medium) to each well, continue to cultivate for 4 h, measure the absorbance at 450 nm with a microplate reader, and Based on this value, the v...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com