A kind of tröger's base-Schiff base derivative and its preparation method and application
A technology of derivatives and anticancer drugs, applied in the field of base-Schiff base derivatives and their preparation, to achieve the effects of low toxicity, readily available raw materials, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1ba
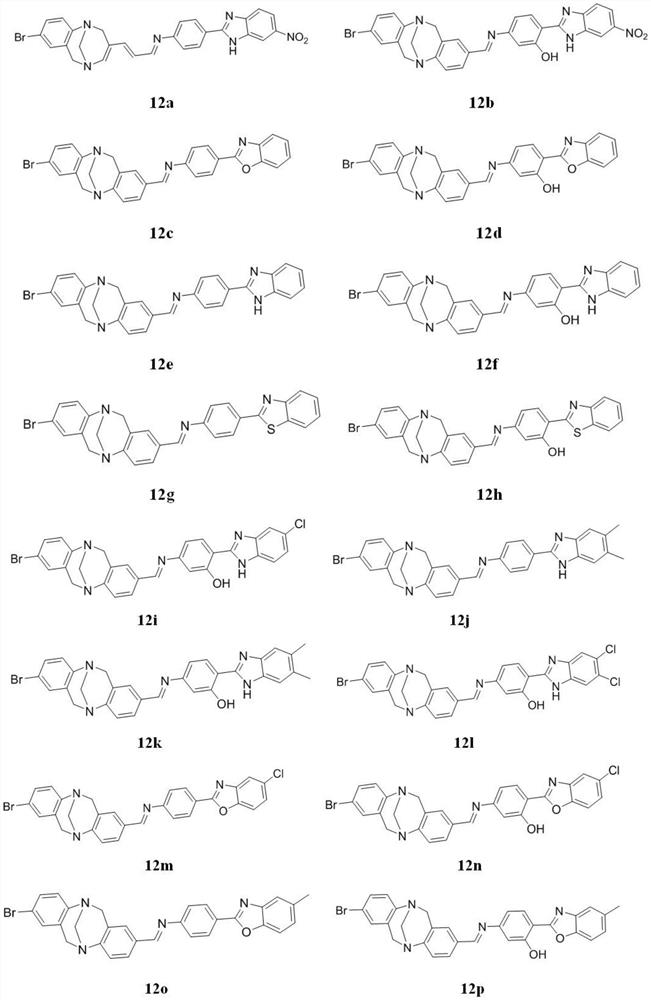
[0044] Example 1 Synthesis of base-Schiff base derivatives 12 and 13
[0045] 1. Synthesis of Intermediate 3
[0046] Weigh 10.32g (60mmol) of p-bromoaniline and 4.5g (150mmol, n=3) of paraformaldehyde into a 250mL round bottom flask and cool to minus 15°C, slowly add 120mL of trifluoroacetic acid dropwise through a constant pressure dropping funnel, After dropping, move the reaction system to room temperature for 7 days, quench with ice water, adjust the pH to neutral with ammonia water, extract with dichloromethane, extract the organic phase with saturated saline, dry over anhydrous sodium sulfate, and perform column chromatography Separation and purification (petroleum ether: ethyl acetate = 5:1), and finally recrystallization with acetone, drying, weighing, and collection to obtain intermediate 3.
[0047] 2. Synthesis of Intermediate 4a
[0048]Weigh 5mmol of the solid compound 3 into a 100mL round bottom flask, add 20mL of dry THF, cool the system to minus 78°C under...
Embodiment 2
[0300] Example 2 The aggregation-inducing effect of each product of the present invention
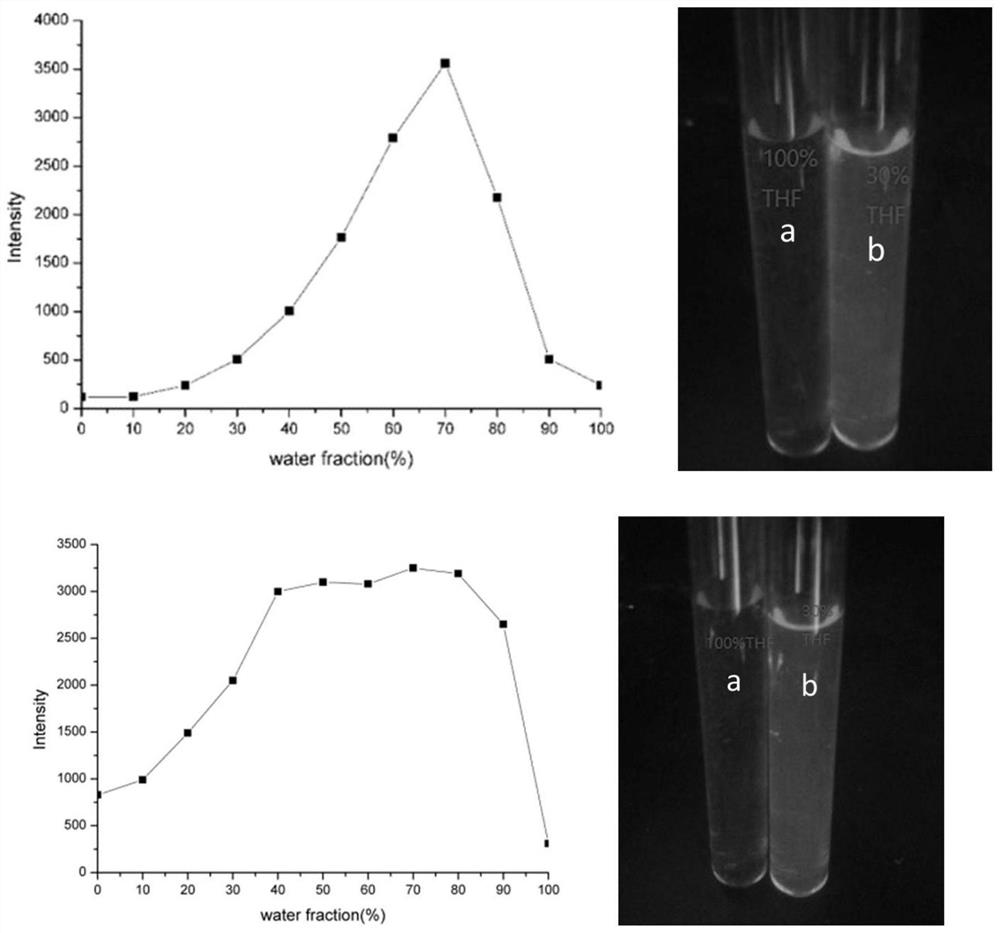
[0301] In this example, compounds 12c and 12o were taken as examples to illustrate the aggregation-inducing effect of the products. with tetrahydrofuran and distilled water according to V DTHF :V H2O Different ratios of 10:0, 9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2:8, 1:9 are used as solvents, compound 12 into 1×10 -6 mol L -1 solution to test the product in different THF-H 2 The relationship between fluorescence intensity and water content in the solvent with O ratio, the test results are as follows figure 1 shown. from figure 1 It can be seen that the present invention The base-Schiff base derivative has excellent luminescence performance, is blue luminescence, has obvious aggregation-induced luminescence and solid-state luminescence, and has application potential in the preparation of new blue solid-state luminescence materials and aggregation-induced blue luminescence materials....
Embodiment 3
[0302] Embodiment 3 metal ion detection
[0303] The metal ion recognition experiment results of each compound are shown in Table 2 below
[0304] Table 2 The fluorescence intensity of the product for the recognition of 7 metal ions
[0305]
[0306] The sign "-" indicates the decrease of relative fluorescence intensity after adding metal ions, and no sign indicates the increase of relative fluorescence intensity after adding metal ions.
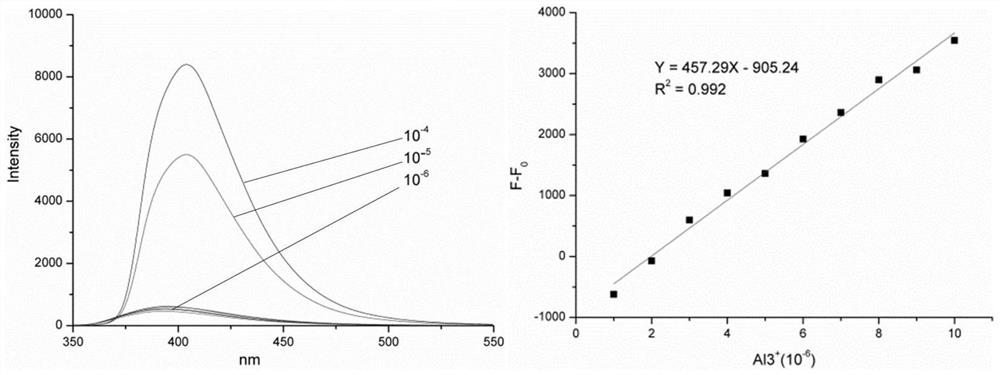
[0307] As can be seen from Table 2, this type of compound has a good effect on Sn 4+ , Fe 3+ 、Al 3+ All have good response, and the relative fluorescence intensity has varying degrees of change. Among them, compound 12d had the most obvious and specific fluorescence intensity after adding metal ions.
[0308] The compound 12d and its intermediate 8 were compared and tested under the same conditions, and the test results are shown in Table 3 below.
[0309] Spectral data of table 3 compound 12d and its intermediate after adding metal...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com