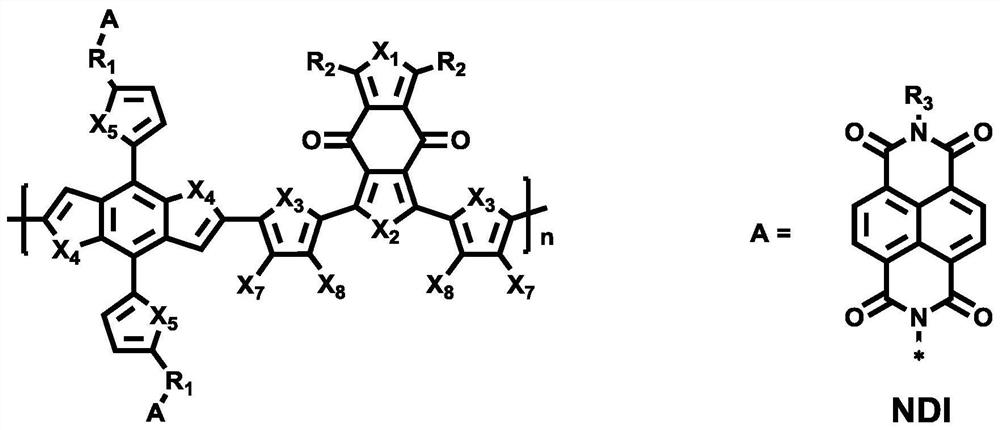
A kind of benzodithiophenedione-benzodithiophene double cable polymer and its preparation and application
A technology of benzodithiophene and benzodithiophene, which is applied in the field of organic semiconductor materials, can solve the problems of limited application, low photoelectric energy conversion efficiency, etc., and achieves good solubility, simple and effective synthesis method, and high absorption range. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Synthesis of benzodithiophene dione-benzodithiophene double cable polymer PClBDBNDI:
[0085] The synthetic reaction formula of double-cable polymer PClBDBNDI is as follows:
[0086]
[0087] Concrete synthetic steps are:
[0088] (1) In a 100mL reaction flask, dialkyl bromide substituted benzodithiophene M 1 -1 (225mg, 0.22mmol) and NDI-1 (213mg, 0.49mmol) were dissolved in 30mL DMF, heated to 80°C, and after the dissolution was balanced, potassium carbonate (152mg, 1.10mmol) was added and reacted for 24 hours. After cooling, add Chloroform 50mL, deionized water 200mL, extraction, liquid separation, the organic phase was washed with brine, dried, and the organic solvent was evaporated under reduced pressure to obtain a crude product. Crude product is separated through silica gel column, and eluent dichloromethane:n-hexane=2:1 (v / v), obtains pure M 2 -1, about 90% yield.
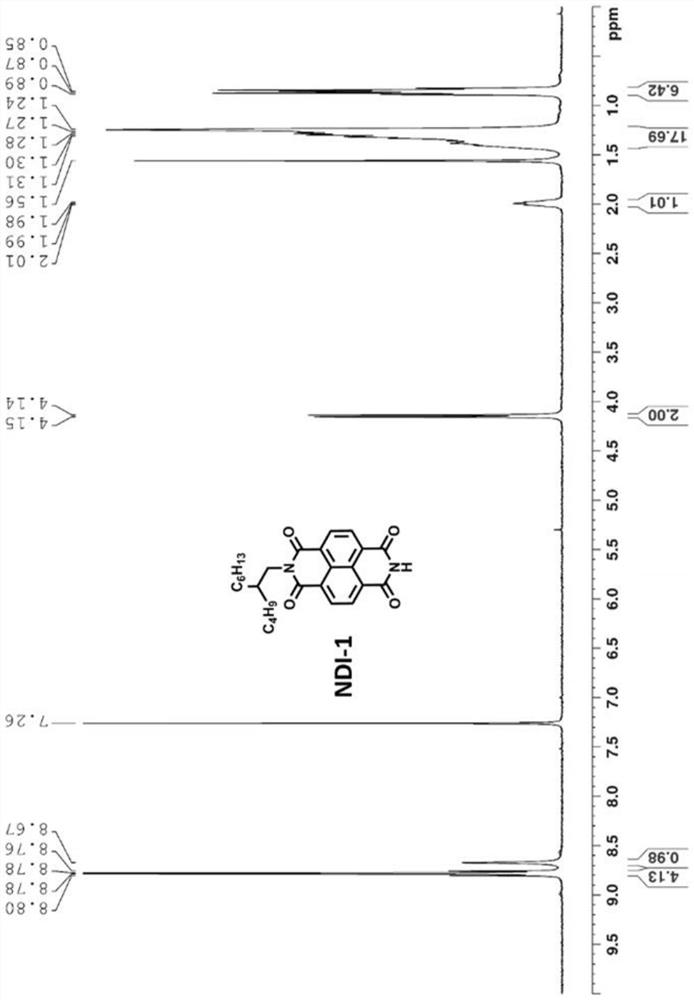
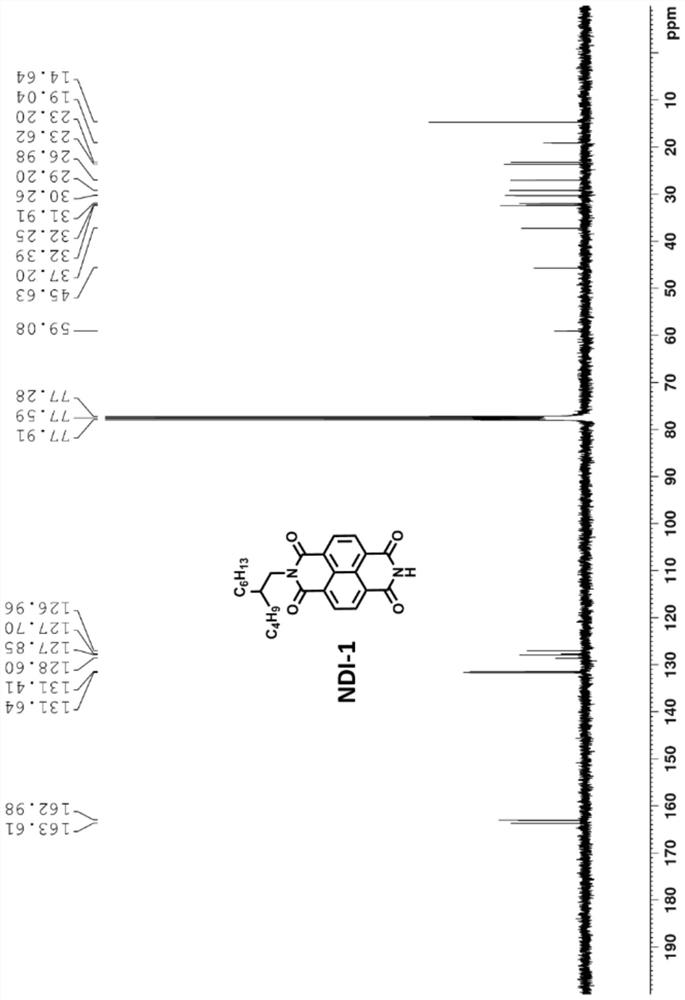
[0089] 1 H NMR (400MHz, CDCl 3 ): δ(ppm)8.75(t,8H),7.56(s,2H),7.20(d,2H),6.88(d,2H),4.18(...
Embodiment 2
[0098] Benzodithiophenedione-benzodithiophene double cable polymer PClBDBNDI single-component organic solar cell device:
[0099] The specific device structure of organic solar cells is shown in the attached Figure 10 Shown: Indium tin oxide / zinc oxide (ITO / ZnO) is used as an electrode on a glass substrate, an organic semiconductor active layer is spin-patterned on it, and molybdenum trioxide / silver (MoO 3 / Ag) as the electrode. The thickness of the zinc oxide layer is about 40nm, the thickness of the active layer is about 70nm, the thickness of the molybdenum oxide is about 10nm, and the thickness of the silver is about 80nm.
[0100] The obtained benzodithiophene dione-benzodithiophene type double-cable polymer PClBDBNDI is applied in non-fullerene solar cells as the only active component, with indium tin oxide / zinc oxide (ITO / ZnO) and Molybdenum trioxide / silver (MoO 3 / Ag) as the electrode. The active layer PClBDBNDI was obtained by spin-coating a 10 mg / mL toluene:DIO=...
Embodiment 3
[0103] NDI-2: 1.2g of 1,4,5,8-naphthalenetetracarboxylic anhydride was dissolved in 50mL of DMF solvent, heated to 120°C under nitrogen atmosphere; 1.08g of 2-butyloctylamine was gradually dropped into the reaction solution , react for 6 to 12 hours, use brine and dichloromethane to extract, wash, and dry; dissolve the crude product in 50 mL of acetic acid, and add 2.7 g of ammonium acetate, react at 100 ° C for 3 to 5 hours, use brine and dichloro Methane was extracted, washed, and dried; the crude product was separated through a silica gel column using dichloromethane as a developing solvent to obtain 1.16 g of pure NDI-2. Its NMR spectrum is as Figure 11 shown. According to the synthesis route as in Example 1, the synthesis of the final double-cable polymer was completed.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com