Preparation method of flame-retardant electrolyte for lithium ion battery
A lithium-ion battery and electrolyte technology, which is applied in the field of preparation of lithium battery flame-retardant electrolytes, can solve the problems of complex synthesis process, high equipment and operation requirements, and high difficulty of lithium hexafluorophosphate, and achieve good thermal stability, temperature resistance, and conditions mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
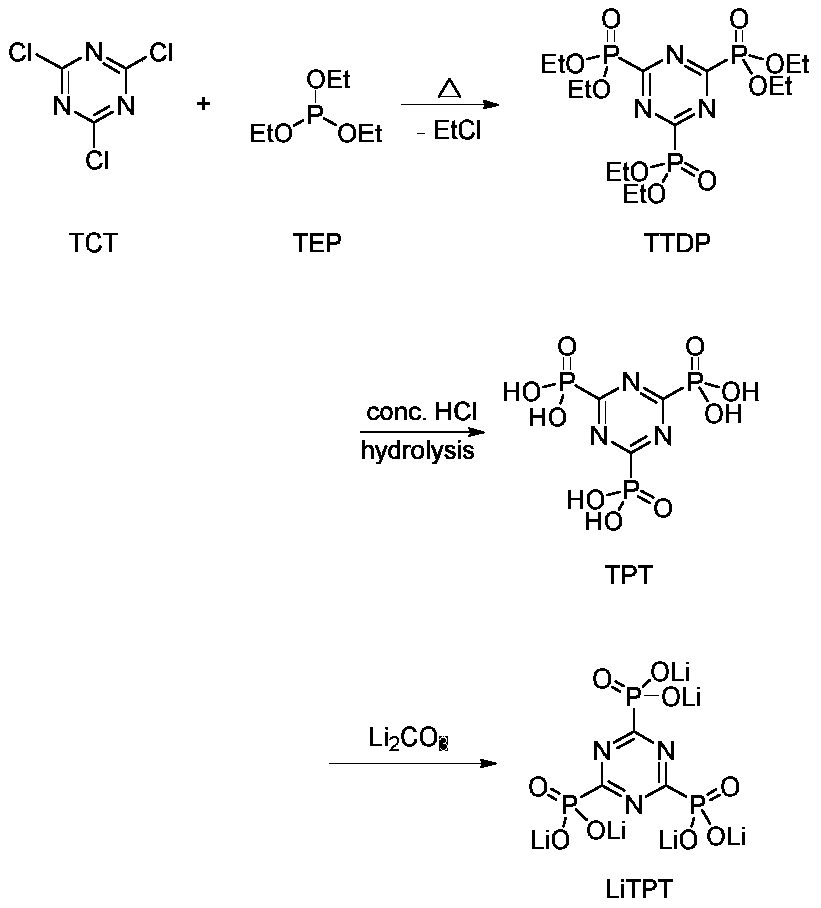
[0021] [Example 1]: Preparation of TTDP. Take 60mL of triethyl phosphite (TEP, 0.34mol) into a 200mL three-neck round bottom flask. Under magnetic stirring, 11.1g of cyanuric chloride (TCT, 0.06mol) was divided into three batches, and slowly added to triethyl phosphite within 1 hour at room temperature, TCT gradually dissolved, the reaction released a lot of heat, and released ethyl chloride alkane gas to obtain a yellow transparent solution. After adding TCT, raise the temperature to 100-105°C for 8 hours, cool to 50°C, slowly add 35mL of petroleum ether (boiling range 60-90°C), continue to stir and cool to room temperature, and stir overnight, a large amount of colorless crystals are precipitated. Suction filtration, wash 3 times with 20mL petroleum ether, remove unreacted TEP and TCT, obtain colorless crystal 22.9g, namely 1,3,5-triazine-2,4,6-triphosphate ethyl ester (TTDP, produced rate: 78%).
[0022] Adopt same reaction step, the productive rate that carries out reac...
Embodiment 2
[0025] [Example 2]: Preparation of TPT. Add 19.6g TTDP (0.04mol) into 130mL concentrated hydrochloric acid, reflux for 36h under magnetic stirring, cool to 50°C, distill off HCl under reduced pressure, concentrate to 40mL, extract three times with 90mL ethyl acetate to remove unhydrolyzed TTDP. The aqueous phase was concentrated to nearly dryness, and dried in a vacuum oven at 120° C. for 12 hours to obtain 12.3 g of a white solid, namely 1,3,5-triazine-2,4,6-triphosphoric acid (TPT, yield: 96% ).
[0026] Using the same reaction steps, but extracting with dichloromethane, benzene, toluene or petroleum ether, the yields were 94%, 89%, 91% and 85%, respectively.
[0027] Using the same reaction steps, reflux in concentrated hydrochloric acid for 12h, distill under reduced pressure at 70°C, and extract with ethyl acetate, the yield is 84%.
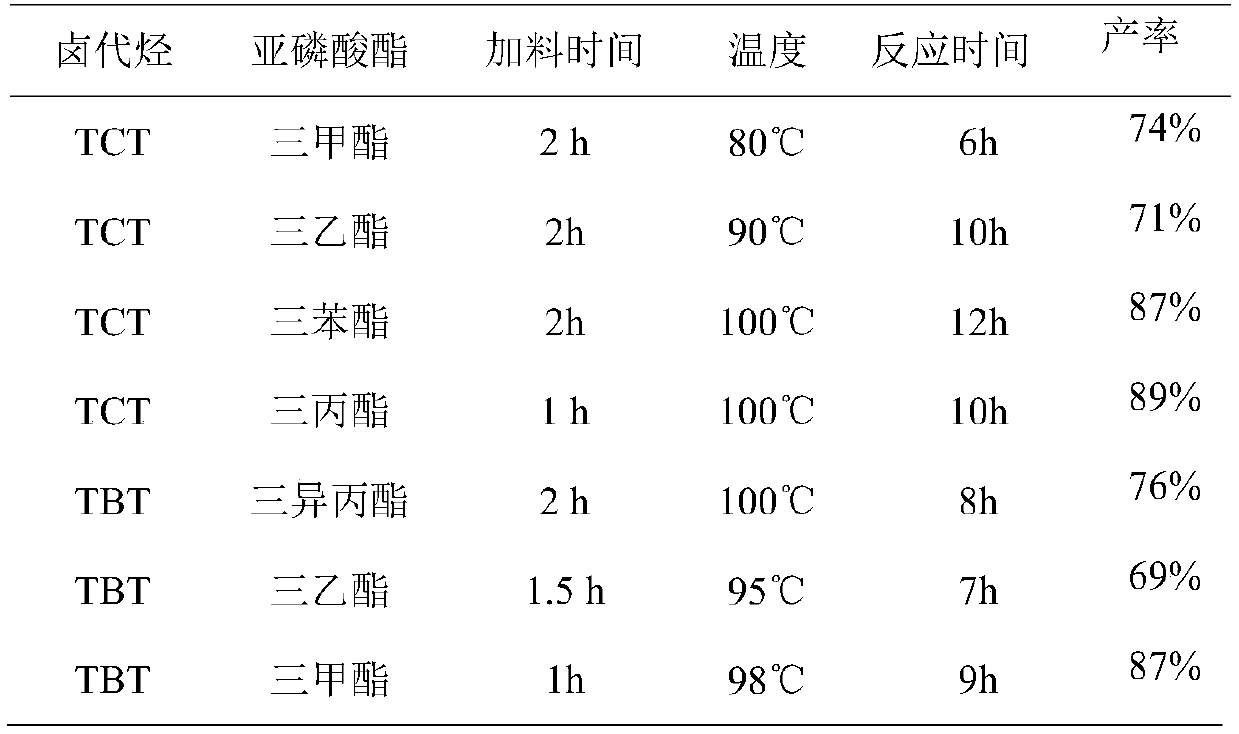
[0028] The hydrolysis of different ester groups of TTDP prepares the productive rate of TPT as shown in Table 2:
[0029] The hydrolysis...
Embodiment 3
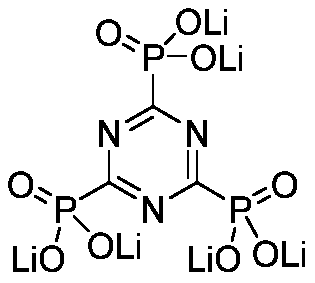
[0031] [Example 3]: Preparation of LiTPT. Take 9.63g TPT (0.03mol) and add it into 20mL deionized water, stir at room temperature for 30min to fully dissolve it. Get 6.65g lithium carbonate (0.09mol) and dissolve in 30mL 0.1mol L -1 dilute hydrochloric acid. The hydrochloric acid solution was added dropwise to the TPT aqueous solution, stirred at room temperature for 12 h, the solvent was concentrated, and vacuum-dried to constant weight to obtain LiTPT solid with a yield of 95%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com