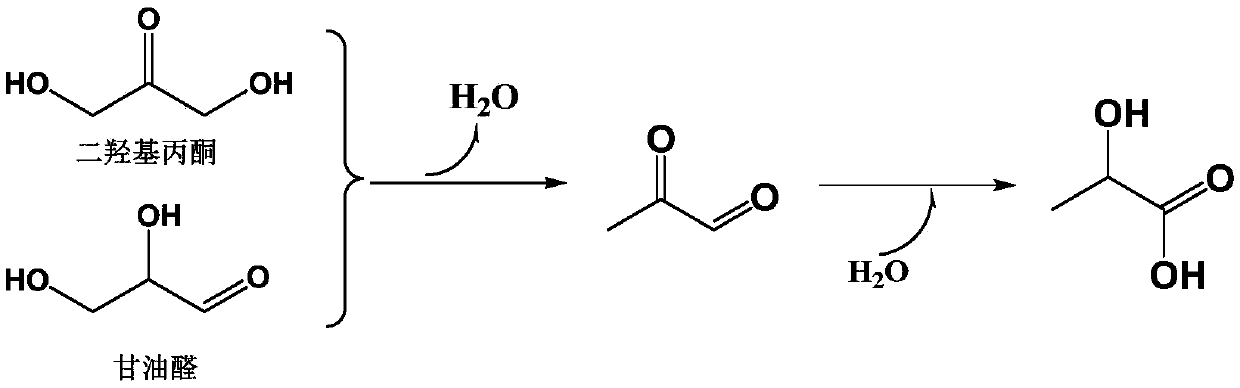
Method for preparing lactic acid
A technology of lactic acid and titanium-silicon molecular sieves, which is applied in the preparation of carboxylate, carbon-based compounds, chemical instruments and methods, etc., can solve the problems that lactic acid cannot be produced and applied on a large scale, the reaction route is long, and the air environment is polluted. Achieve the effect of being suitable for large-scale industrial production application, high conversion rate and lactic acid yield, safe and efficient process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0046] This preparation example prepares Sn-MFI molecular sieve, and specific preparation method is:
[0047] tin tetrachloride pentahydrate (SnCl 4 .5H 2 O) Dissolve in water, add this aqueous solution to tetraethyl orthosilicate (TEOS) and stir, add tetrapropylammonium hydroxide (TPAOH, 20% aqueous solution) and water under stirring, and continue stirring for 30 minutes to obtain a chemical composition of 0.03SnO 2 : SiO 2 :0.45TPA: 35H 2 The clear liquid of O was then crystallized at 433K for 2 days, and then the obtained solid was filtered, washed with distilled water, dried at 393K for 5 hours, and then calcined at 823K for 10 hours to obtain a molecular sieve sample. Among them, the amount of TEOS is 15.31g, the amount of TPAOH is 33.67g, SnCl 4 .5H 2 The amount of O used is 0.38g, and the amount of water used is 39.64g.
preparation Embodiment 2
[0049] This preparation example references "Nemeth L, Moscoso J, Erdman N, et al.Synthesis and characterization of Sn-Beta as a selective oxidation catalyst [J]. Studies in Surface Science & Catalysis, 2004, 154 (04): 2626-2631" Method prepares Sn-Beta molecular sieve, the preparation method of the adopted Sn-Beta molecular sieve is:
[0050] tin tetrachloride pentahydrate (SnCl 4 .5H 2 O) Dissolve in water, add tetraethyl ammonium hydroxide (TEAOH) to this aqueous solution and stir with tetraethyl ammonium hydroxide (TEAOH), stir until TEOS evaporates to obtain alcohol, add hydrogen fluoride (HF) to the clarified liquid , forming a paste-like thin layer. Finally, a suspension of dealuminated nano Beta seeds (20nm) and water is added to obtain a chemical composition of 0.03SnO 2 : SiO 2 :6TEA:15H 2 O: 10HF clear liquid, then crystallized at 413K for 10 days, then filtered the obtained solid, washed with distilled water, dried at 393K for 5 hours, and then roasted at 823K ...
preparation Embodiment 3
[0052] References for this preparation example "Yang X, Wu L, Wang Z, et al. Conversion of dihydroxyacetone to methyl lactate catalyzed by highly active hierarchical Sn-USY at room temperature [J]. Catalysis Science & Technology, 2016, 6 (6): 1757- 1763" method to prepare Sn-USY molecular sieve, the preparation method of the adopted Sn-USY molecular sieve is:
[0053] H-USY molecular sieve was mixed with nitric acid, treated at 85°C for 8h, the sample was filtered and washed with deionized water, and dried at 120°C for 12h to obtain a solid sample. This solid sample was mixed with tin tetrachloride pentahydrate (SnCl 4 .5H 2 O) mixed for 1h to obtain a chemical composition of 0.03SnO 2 : 100SiO2 2 The mixed liquid was dried at 100°C for 12 hours, and finally calcined at 550°C for 3 hours to obtain a molecular sieve sample. Among them, the amount of H-USY is 2.0g, the amount of nitric acid is 50mL, the amount of SnCl 4 .5H 2 The amount of O used is 0.6 g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| flash point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com