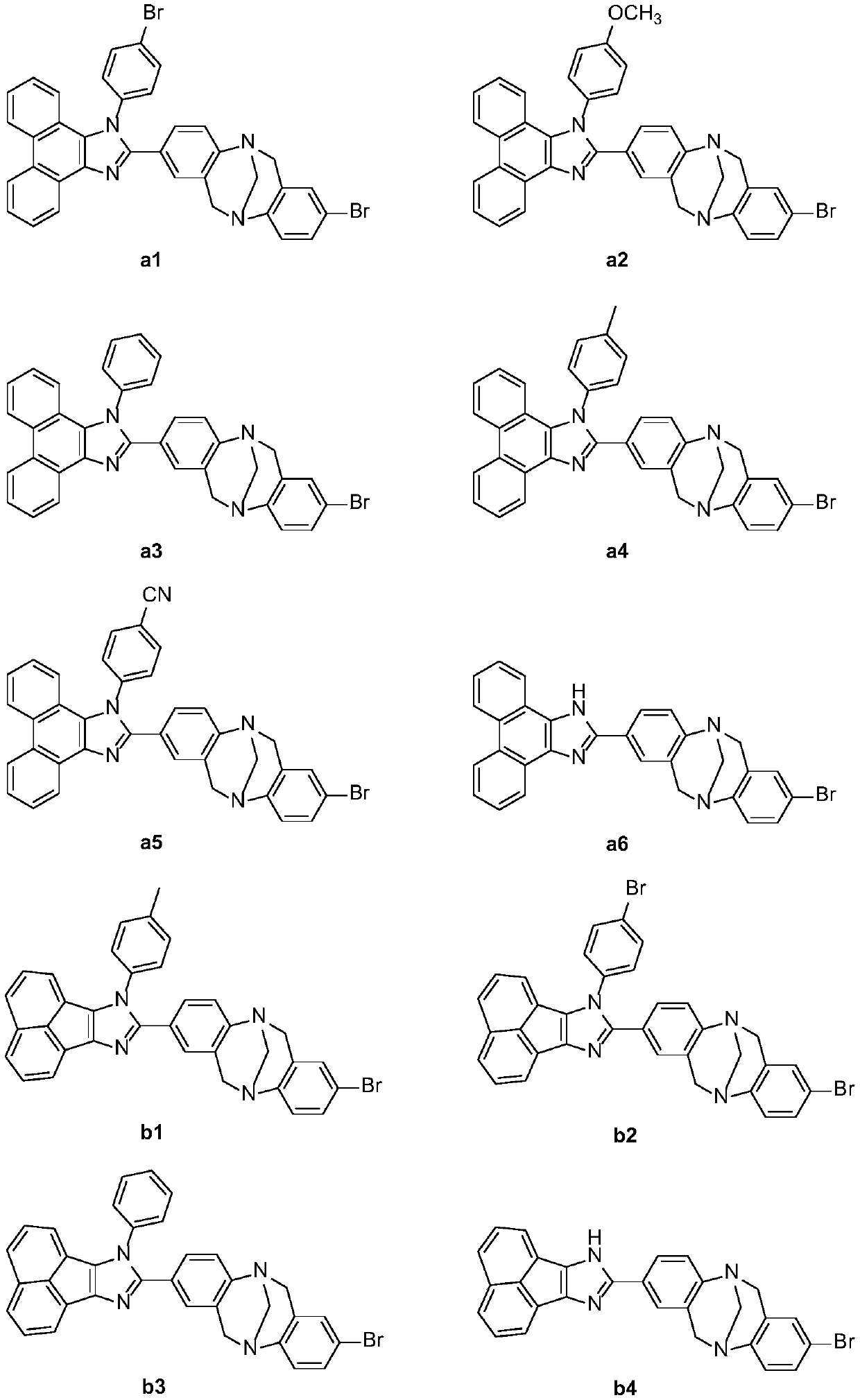
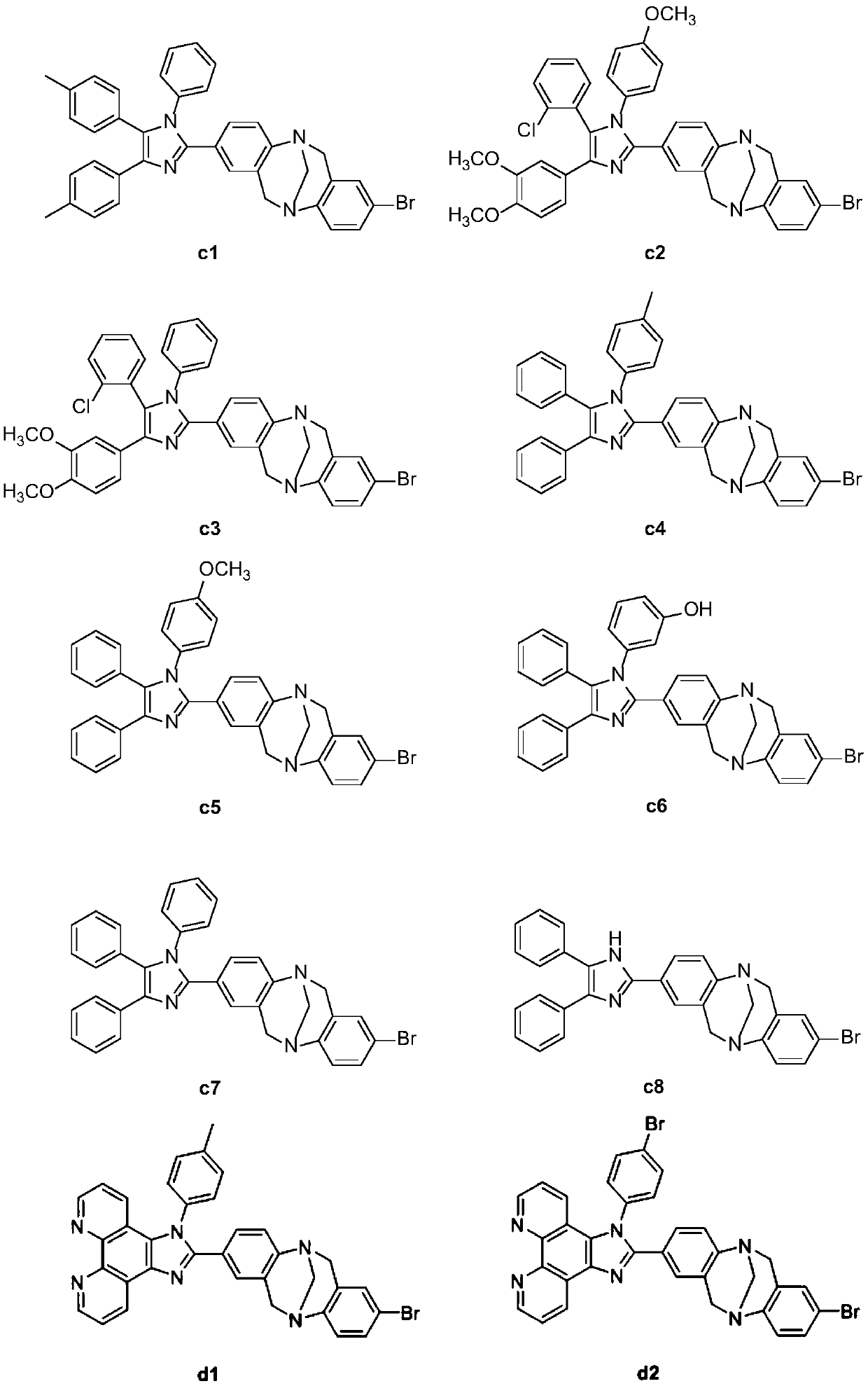
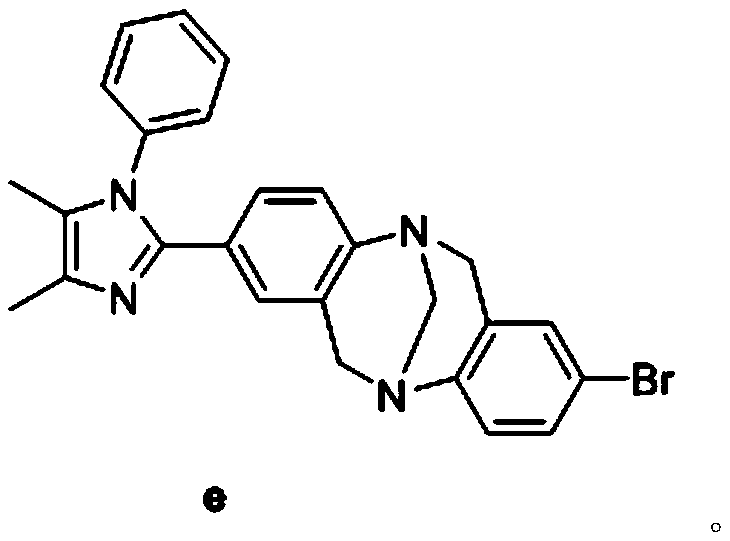
Novel Tr*ger's Base-imidazole derivatives and preparation method and application thereof
An imidazole derivative, a new type of technology, applied in chemical instruments and methods, drug combinations, organic chemistry, etc., to achieve the effects of convenient post-processing, mild conditions, and good inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1 new type Synthesis of Base-imidazole derivatives
[0033] 1. Synthesis of Intermediate 1
[0034] Weigh 10.32g (60mmol) of p-bromoaniline and paraformaldehyde (n=3,150mmol) into a 250mL round-bottomed flask and cool to minus 15°C, slowly add 120mL of trifluoroacetic acid dropwise through a constant pressure dropping funnel. The reaction system was moved to room temperature for seven days, quenched with ice water, adjusted to neutral pH with ammonia water, extracted with dichloromethane, and the organic phase was extracted with saturated brine, dried over anhydrous sodium sulfate, separated and purified by column chromatography (V 石油醚 :V 乙酸乙酯 =5:1), and finally recrystallized with acetone, dried, weighed, and collected to obtain intermediate 1.
[0035] 2. Synthesis of Intermediate 3
[0036] Weigh 5 mmol of the solid compound 3 into a 100 mL round-bottomed flask, add 20 mL of dry-treated tetrahydrofuran, cool the system to minus 78 °C under anhydrous a...
Embodiment 2
[0299] Example 2 The aggregation-inducing effect of each product of the present invention
[0300] In this example, compounds c4 and a3 are taken as examples to illustrate the aggregation-inducing effect of the products. with tetrahydrofuran and distilled water according to V DTHF :V H2O 10:0, 9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2:8, 1:9 are used as solvents in different ratios, and the compound is formulated as 1 ×10 -6 mol L -1 solution to test the product in different THF-H 2 The relationship between the fluorescence intensity and the water content in the solvent with O ratio, the results show that the novel The Base-imidazole derivatives emit blue light, have obvious aggregation-induced luminescence and solid-state luminescence, and have application potential in the preparation of new blue solid-state luminescent materials and aggregation-induced blue light materials.
Embodiment 3
[0301] Embodiment 3 thermogravimetric analysis
[0302] The thermal stability of the compound was tested by a thermogravimetric analyzer, the heating rate was 10° C. / min, and the test range was 0-400° C. under nitrogen. The results show that the temperature for compound a4 to lose 5% of its weight is 180°C, and when the temperature reaches 200°C, the compound begins to decompose, and the temperature for compounds c2, a3, and c7 to lose 5% of their weight is about 200°C, and it does not start until the temperature reaches 350°C break down. The longer the conjugated system of the molecule, the higher the decomposition temperature, which is in line with the general rule. The results show that this type of compound has good thermal stability, meets the basic requirements of device materials, and has certain application potential.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com