Polyamino acid carrier with acid-sensitive connecting arm in middle as well as preparation method and application of polyamino acid carrier
A polyamino acid and connecting arm technology, applied in the field of polyamino acid carrier, can solve the problem of easily damaged delivery protein, and achieve the effect of stable delivery and precise regulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The preparation of polyamino acid carrier I:
[0052] Step 1: 4-(4-Hydroxybenzyl)oxazolidine-2,5-dione (III-1)
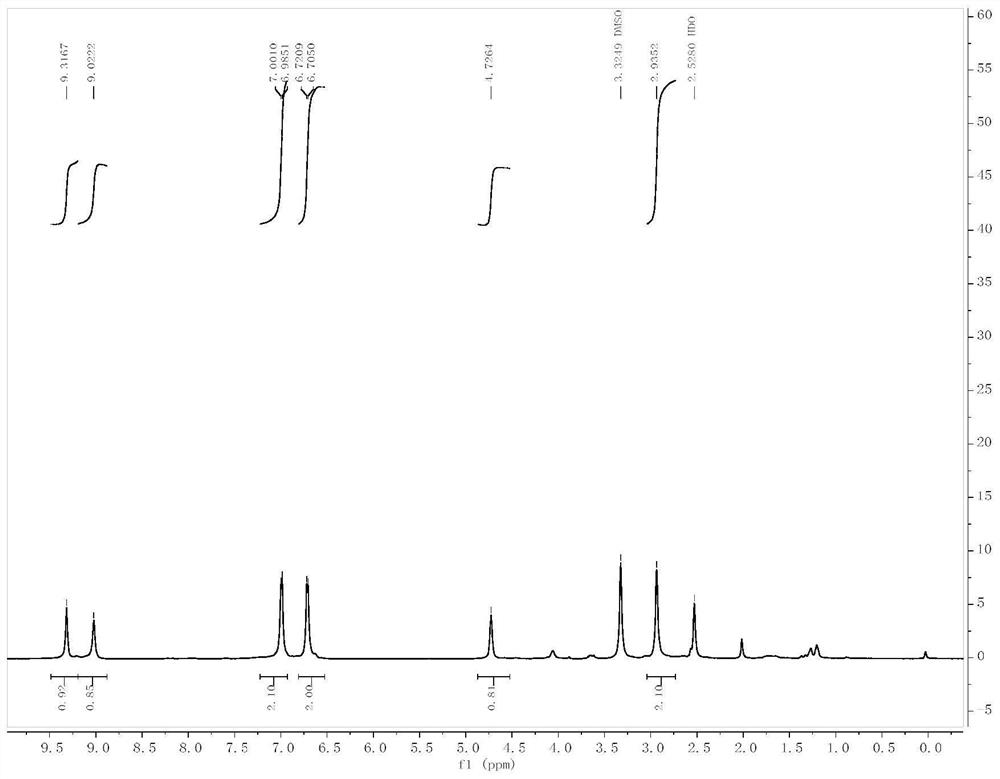
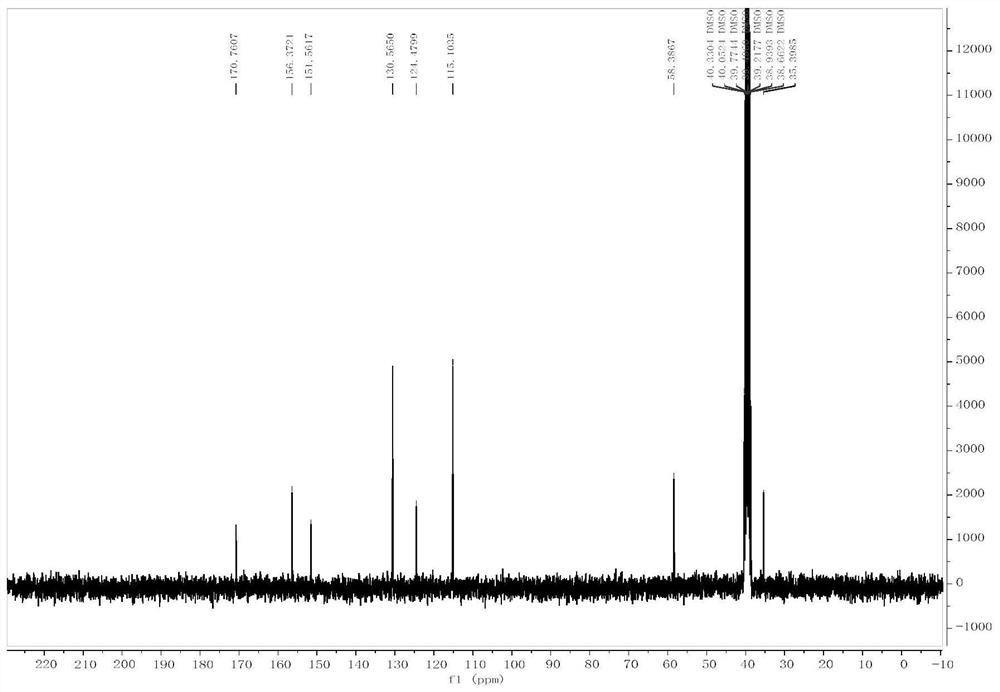
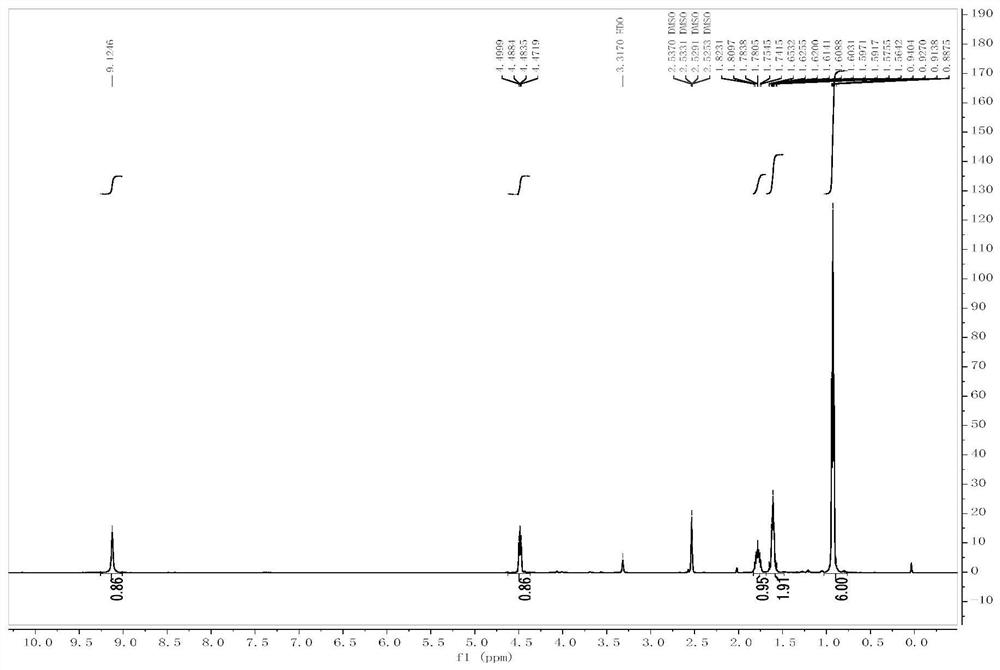
[0053] Add L-tyrosine (II-1, 1g, 5.52mmol), triphosgene (3.28g, 11.04mmol) and 10mL anhydrous tetrahydrofuran to a 100mL three-necked flask in sequence, and heat under reflux for 12h under nitrogen protection. TLC (petroleum ether : Ethyl acetate=1:1) to monitor the completion of the raw material reaction. The reaction solution was cooled to room temperature, and the solvent was distilled off under reduced pressure to obtain a colorless oil. The crude product was purified by column chromatography (petroleum ether: ethyl acetate = 4:1-1:1 gradient elution) to obtain 600 mg of white solid, yield: 52.5%. ESI-MS(m / z):206.1[M-H] - . m.p.138-140°C. 1 H-NMR (300MHz, DMSO), δ (ppm): 9.31 (s, 1H, Oh ),9.02(s,1H, NH ),6.98-7.00(d,2H,J=4.5Hz, ArH ),6.70-6.72(d,2H,J=4.5Hz, ArH ),2.93(m,3H,Ar CH 2 CH ). 13 C-NMR (75MHz, DMSO) δ 170.76, 156.37, 151.56, 130...
Embodiment 2
[0069] Preparation of polyamino acid carrier chemically bonded protein preparation:
[0070] Step 1: Add 4mg ribonuclease A (RNase-A) to 5mL PBS buffer solution, add 0.1mg inorganic weak base salt after stirring at room temperature for 10min (weak base salt can be: sodium bicarbonate (NaHCO 3 )) continue to stir for 20min.
[0071] Step 2: Add 10mg of polyamino acid polymer carrier I into 10mL of organic solvent (the organic solvent can be methanol, ethanol, acetonitrile, tetrahydrofuran, acetone or any mixed solvent of the two), sonicate for 10 minutes and stir at room temperature after it is completely dissolved.
[0072] Step 3: Add 0.1mg I 2 Dissolve in 1mL organic solvent (the organic solvent can be methanol, ethanol, acetonitrile, tetrahydrofuran, acetone or any mixed solvent of the two), and store away from light
[0073] Step 4: slowly drop the mixed solution obtained in step 1 into the mixed solvent in step 2, and control the dropping rate to 2 drops per second. Af...
Embodiment 3
[0077] Agarose gel electrophoresis of RNase-A
[0078] RNA agarose electrophoresis was used to assess GSH-triggered protein release and restoration of protein viability. 20 μg / mL of RNA and I@RNase-A (the concentration of RNase-A was 40 μg / mL) were incubated with different concentrations of GSH (0, 5 μM, 50 μM, 500 μM and 5 mM) at 37 °C. Then, the mixture was analyzed by 8% agarose gel electrophoresis at a constant voltage of 120 V for 20 minutes. Electropherogram as Figure 20 As shown, GSH-triggered protein release and recovery can be seen well.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com