Fluorescent probe HM, and preparation method and application thereof
A fluorescent probe and stock solution technology, applied in the field of fluorescent probes, can solve the problems of complex operation, low sensitivity, and inability to use HClO/ClO-detection, etc., and achieve the effects of good selectivity, high sensitivity and short response time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The preparation of embodiment 1 fluorescent probe
[0028] (1) Dissolve 7-(diethylamino)coumarin-3-carbaldehyde (0.1225g, 0.5mmol) and 1-(2-hydroxy-5-methoxyphenyl)ethanone (0.083,0.5mmol) In acetonitrile (10mL), piperidine (0.1mL, 1mmol) was added dropwise, heated to reflux for 20 hours, during which a red solid was continuously precipitated;
[0029] (2) After the reaction finishes, cool to room temperature, and a large amount of red solids are precipitated;
[0030] (3) Filtrate under reduced pressure, wash the precipitate, and dry to obtain 0.063 g of a red solid product with a yield of 32%.
[0031] For fluorescent probes 1 H NMR characterization, the results are as follows:
[0032] 1 H NMR (600MHz, DMSO-d 6 ,δ / ppm): δ11.98(s,1H),8.55(s,1H),8.07(d,J=15.4Hz,1H),7.77(d,J=15.3Hz,1H),7.52(d, J=9.0Hz, 1H), 7.44(d, J=3.0Hz, 1H), 7.21(dd, J=9.0, 3.0Hz, 1H), 6.95(d, J=9.0Hz, 1H), 6.82(dd, J=9.0,2.2Hz,1H),6.62(d,J=1.9Hz,1H),3.80(s,3H),3.50(q,J=7.0Hz,4H),1.15(t,J=7.0...
Embodiment 2
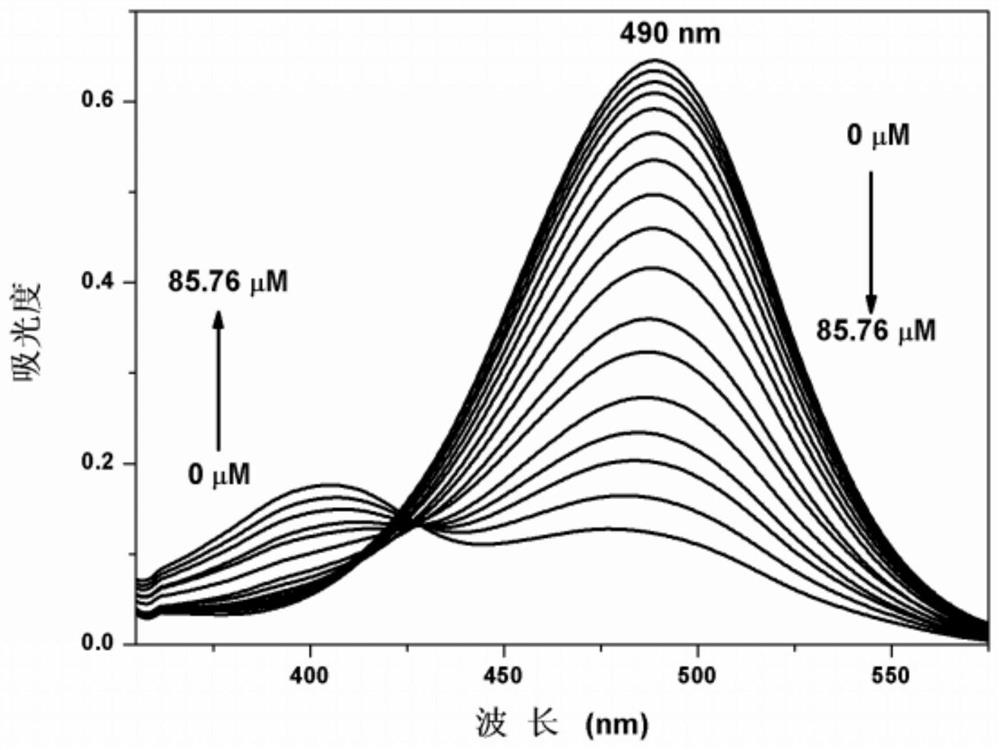
[0034] Embodiment 2 fluorescent probe HM along with ClO - Varying UV Absorption Spectrum
[0035] In 2.0mL C 2 h 5 Add 10.0 μL fluorescent probe stock solution to OH / PBS (2 / 3, v / v) system, and carry out ClO - UV titration experiment, and record its UV absorption spectrum ( figure 1 ). With ClO - With the increase of the amount, the ultraviolet absorption value at 400nm increases, and the ultraviolet absorption value at 490nm decreases.
Embodiment 3
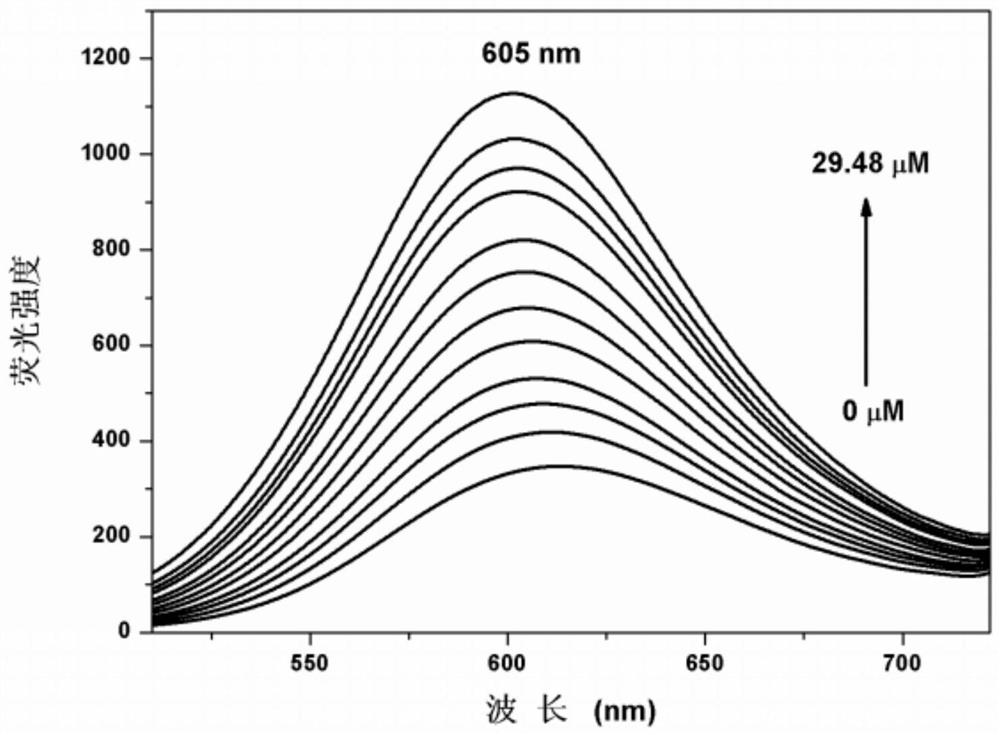
[0036] Embodiment 3 fluorescent probe HM along with ClO - Fluorescence titration plot of change
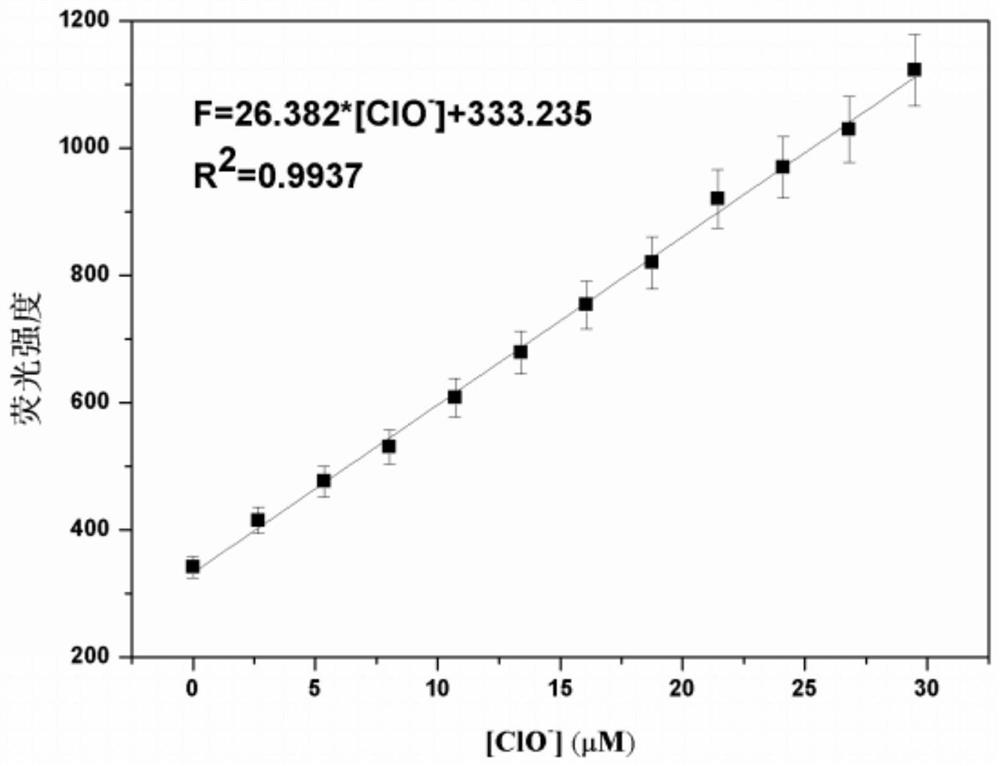
[0037] In 2.0mL C 2 h 5 Add 10.0 μL fluorescent probe stock solution to OH / PBS (2 / 3, v / v) system for ClO -Fluorescence titration experiment, detected on a spectrofluorometer, with ClO - With the increase of , the fluorescence intensity at 605nm is gradually enhanced ( figure 2 ). Instrument parameters: the slit widths of the excitation wavelength and emission wavelength are 5.0nm and 5.0nm respectively, the voltage is 600V, and the maximum excitation wavelength λ of the fluorescent probe solution ex 485nm, the maximum emission wavelength λ em 605nm. Draw the graph with the fluorescence intensity value F as the ordinate, and get ClO - The working curve of concentration, the linear regression equation is F=26.382*[NaClO]+333.235, the unit of NaClO concentration is 10 -6 mol / L; the linear correlation coefficient is R 2 =0.9937, the best linear response range is 0μM–29.48μM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com