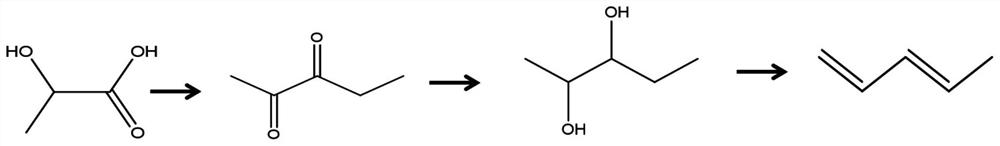
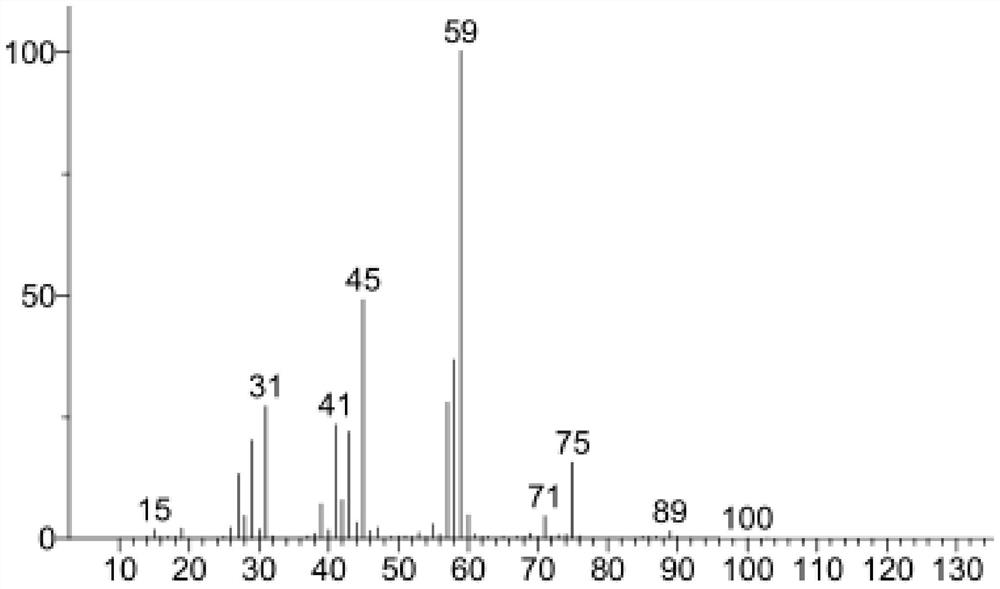
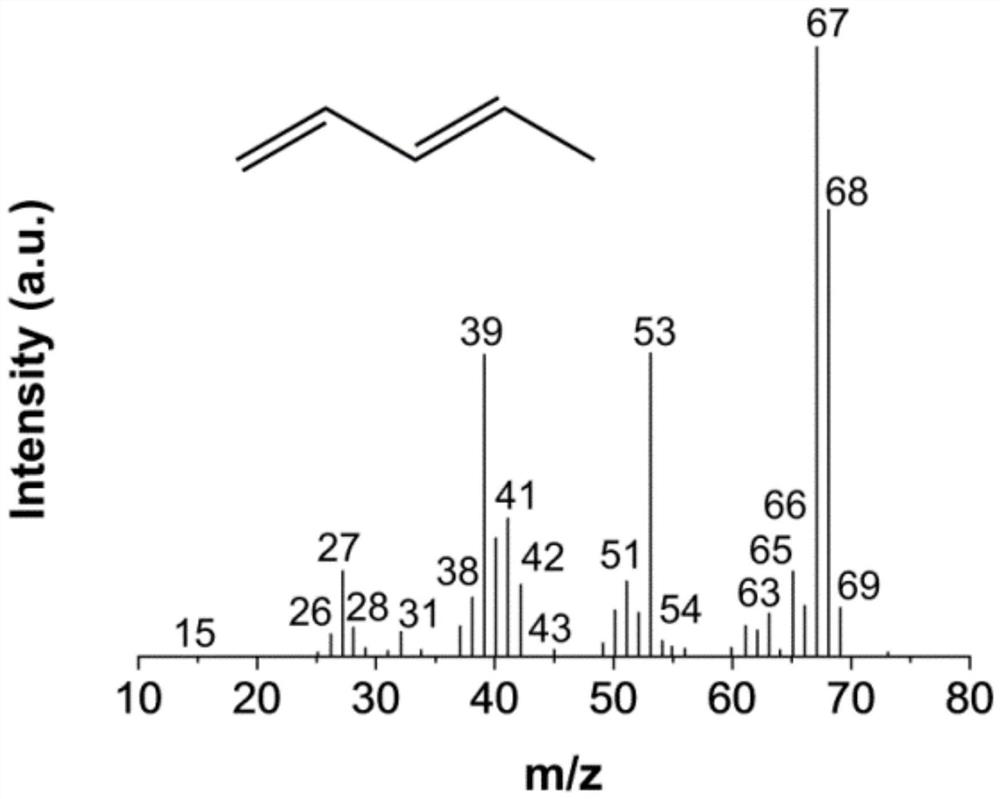
A kind of synthetic method of pentanediol and a kind of synthetic method based on lactic acid conversion to prepare biomass baseline linear pentadiene
A synthesis method, pentadiene technology, applied in the direction of condensation preparation of carbonyl compounds, carbon-based compound preparation, hydroxyl compound preparation, etc., can solve the problems of linear pentadiene with little industrial value, low catalytic efficiency, poor process flexibility, etc. , to achieve good conversion effect, easy raw materials and catalysts, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0086] The invention provides a kind of synthetic method of pentanediol, comprising the following steps:
[0087] 1) The mixed solution after mixing pentanedione, a hydrogenation catalyst and an organic solvent is subjected to a hydrogenation reaction to obtain pentanediol in an atmosphere containing hydrogen.
[0088] In principle, the present invention has no special restrictions on the amount of the hydrogenation catalyst, and those skilled in the art can select and adjust according to practical application needs, product requirements and quality requirements. For efficiency, the concentration of the hydrogenation catalyst in the mixed solution is preferably 1-30 mg / ml, more preferably 6-25 mg / ml, and more preferably 11-20 mg / ml.
[0089] In principle, the present invention does not limit the amount of pentanedione in the mixed solution. Those skilled in the art can select and adjust according to practical application needs, product requirements and quality requirements. Th...
Embodiment 1
[0205] (1) reaction of lactic acid condensation to prepare pentanedione:
[0206] Weigh 2.0g of sodium bicarbonate to prepare 5ml of solution, add 5g of MgO under stirring, let stand for 12h, and dry at 120°C for 10h. Before use, MgO was calcined at 550 °C for 4 h to obtain a condensation catalyst. Weigh 1.5 g of the catalyst and put it into the fixed-bed reactor; before the reaction, the catalyst is at 550 ° C, and the treatment time is preferably 3 h; during the reaction, the feed rate of raw materials is 0.06 ml / min, the flow rate of nitrogen gas is 60 ml / min, and the reaction temperature is 380 °C. The yield of the obtained pentanedione was 73%, and the conversion rate of the raw material was 89%.
[0207] (2) pentanedione hydrogenation to produce pentanediol reaction:
[0208] will Al 2 O 3 After the carrier was calcined at 500°C for 5h, 3.0g was weighed into a beaker, 3ml of chloroplatinic acid solution was added, stirred for 10min, left to stand overnight, dried in...
Embodiment 2
[0212] (1) reaction of lactic acid condensation to prepare pentanedione:
[0213]Weigh 1.8g potassium bicarbonate and configure it into 5ml solution, add 5g Al under stirring condition 2 O 3 , stand for 12h, and dry at 120°C for 10h. Al before use 2 O 3 It was calcined at 600°C for 5h to obtain a condensation catalyst. Weigh 2.0 g of the catalyst and load it into the fixed-bed reactor; before the reaction, the catalyst is kept at 500 ° C, and the treatment time is preferably 4 h; during the reaction, the feed rate of raw materials is 0.08 ml / min, the flow rate of nitrogen gas is 60 ml / min, and the reaction temperature is 300 °C. The yield of pentanedione obtained was 62%, and the conversion rate of raw materials was 93%.
[0214] (2) pentanedione hydrogenation to produce pentanediol reaction:
[0215] ZrO 2 After the carrier was calcined at 500°C for 5h, 3.0g was weighed into a beaker, 3ml of cerium nitrate solution was added, the loading of cerium nitrate was controll...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com