Whole blood perfusion adsorbent as well as preparation method and application thereof
An adsorbent and perfusion technology, applied in the field of natural polymer biomedical materials, can solve the problems of non-degradation, low specific adsorption capacity, side reactions, etc., and achieve good biocompatibility, blood compatibility, and biocompatibility. Strong sex and blood compatibility, the effect of avoiding structural damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The preparation of embodiment 1 whole blood perfusion adsorbent
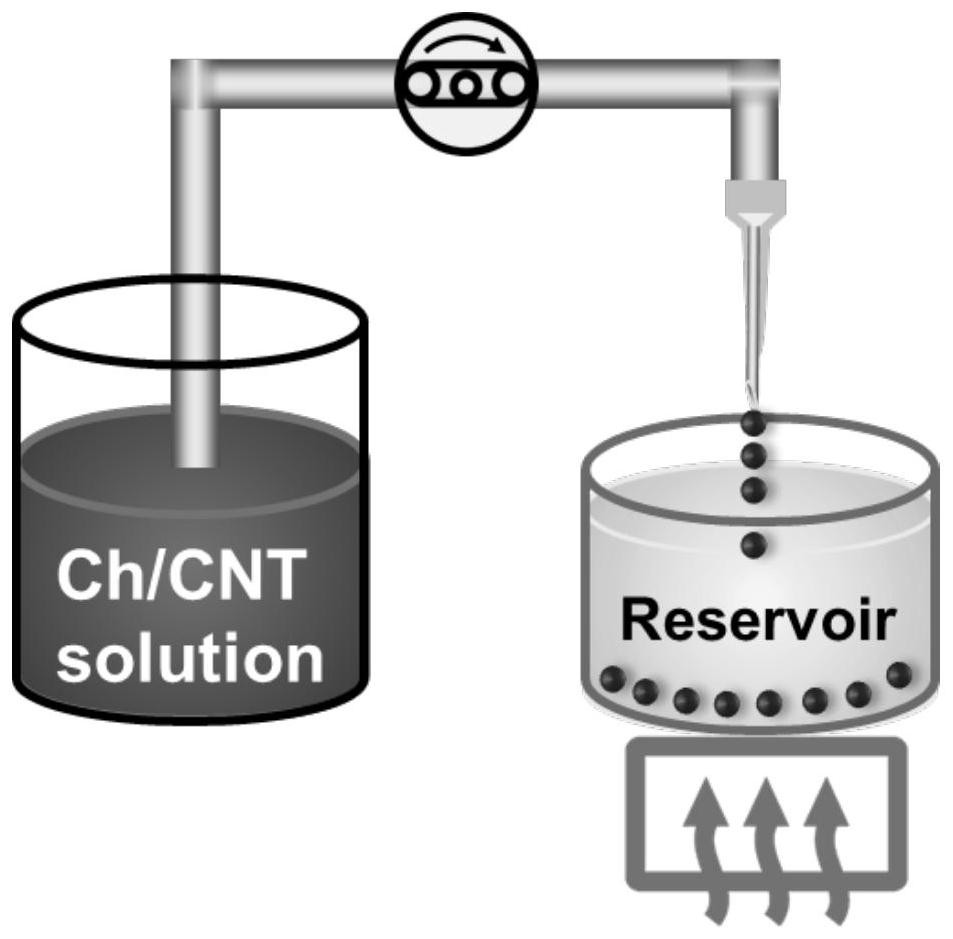
[0048] The preparation method of chitin / alkali / urea aqueous solution is the prior art, and it is used according to the method disclosed in ZL200310111566.3. The preparation method of acidified carbon nanotubes and chitin / carbon nanotube composite solution is the prior art, which is used according to the method disclosed in ZL201610907056.4. Put a certain amount of chitin / carbon nanotube composite solution into the program-controlled equipment, and the schematic diagram of the injection method is as follows: figure 1 As shown; use the program to control the syringe pump, the program is set to two-step method, the first step is perfusion, the speed is 0.2mL / min; the second step is extraction, the speed is 0.02mL / min, the diameter of the injection needle is 0.2mm, Keep the temperature near the sample below 10°C. Drop the solution into a 28% sodium chloride coagulation bath and control the temperature at 60...
Embodiment 2
[0049] Embodiment 2 Preparation of whole blood perfusion adsorbent
[0050] The preparation method of chitin / alkali / urea aqueous solution is the prior art, and it is used according to the method disclosed in ZL200310111566.3. The preparation method of acidified carbon nanotubes and chitin / carbon nanotube composite solution is the prior art, which is used according to the method disclosed in ZL201610907056.4. Put a certain amount of chitin / carbon nanotube composite solution into the program-controlled equipment, use the program to control the syringe pump, the program is set as a two-step method, the first step is perfusion, the speed is 0.5mL / min; the second step is extraction mode, the speed is 0.02mL / min. Keep the temperature near the sample below 10°C. Drop the solution into the coagulation bath of 20% sodium chloride / phytic acid mixture, wait to form large-sized nanofiber microspheres at room temperature, sieve with a 20-mesh screen, put the microspheres into absolute et...
Embodiment 3
[0051] Example 3 Preparation of Adsorbent for Whole Blood Perfusion
[0052]The preparation method of chitin / alkali / urea aqueous solution is the prior art, and it is used according to the method disclosed in ZL200310111566.3. Add a certain amount of zeolite to the chitin solution after grinding, and make a chitin / zeolite composite solution with a ratio of 1:1, put it into the program-controlled equipment, use the program to control the injection pump, and the program is set as a two-step method , the first step is the perfusion method, the speed is 0.5mL / min; the second step is the extraction method, the speed is 0.02mL / min. Keep the temperature near the sample below 10°C. Drop the solution into a 20% to 30% sodium chloride coagulation bath, and control the temperature at 30°C. After forming large-sized nanofiber microspheres, sieve them through a 20-mesh sieve, put the microspheres into absolute ethanol and let them stand After 12 hours, it was washed repeatedly with 50% et...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com