Semi-solidified nutritional agent for solving problem of enteral nutrition intolerance and preparation method of semi-solidified nutritional agent
An enteral nutrition and semi-curing technology, which is applied to the functions of food ingredients, food ingredients as thickeners, food ingredients containing inorganic compounds, etc., can solve problems such as troublesome use, easy to cause diarrhea, vomiting, disappearance, etc., to avoid Intolerance, the effect of suppressing gastroesophageal reflux
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] A semi-solidified nutrient for solving enteral nutrition intolerance, comprising the following components in parts by weight:
[0065] Maltodextrin 500 parts, Whey Protein Isolate 69 parts, Soy Protein Isolate 69 parts, High Oleic Sunflower Oil Powder 30 parts, Soybean Oil Powder 28 parts, Flaxseed Oil Powder 29 parts, Low Methoxyl Pectin 28 parts , 1.6 parts of flavoring agent, 0.1 part of sucralose, 0.5 parts of vitamin complex and 30 parts of mineral complex.
[0066] Wherein, the flavoring agent is one or a combination of milk flavoring, fruit flavoring, vegetable flavoring, and nut flavoring; the flavoring agent in this embodiment is peanut flavoring.
[0067] In parts by weight, the vitamin complex includes: 0.02 part of vitamin A acetate, 0.02 part of cholecalciferol, 0.03 part of dl-α-tocopheryl acetate, 0.006 part of phytonadione, 0.1 part of niacin thiamine, riboflavin 0.1 part of vitamin, 0.008 part of pyridoxine hydrochloride, 0.003 part of cyanocobalamin, ...
Embodiment 2
[0078] A semi-solidified nutrient for solving enteral nutrition intolerance, comprising the following components in parts by weight:
[0079] 643 parts of maltodextrin, 150 parts of protein, 95 parts of fat, 10 parts of low methoxy pectin, 1 part of food flavor, 0.1 part of sucralose, 0.5 parts of vitamin complex and 40 parts of mineral complex.
[0080] Specifically, the protein is composed of 65 parts of whey protein isolate, 65 parts of soybean protein isolate and 20 parts of soybean oligopeptide; the fat is composed of 15 parts of medium chain triglyceride powder, 25 parts of high oleic sunflower oil powder, Composed of 25 parts soybean oil powder and 30 parts rapeseed oil powder;
[0081] Wherein, the flavoring agent is one or a combination of milk flavoring, fruit flavoring, vegetable flavoring, nut flavoring; the flavoring agent in this embodiment is composed of milky flavoring and jujube flavoring.
[0082] In parts by weight, the vitamin complex includes: 0.05 part o...
Embodiment 3
[0093] The preparation method of the product semi-solidified nutrient in this implementation is the same as that of Example 1, except that the mass fraction of the low-methoxyl pectin in the semi-solidified nutrient is 1.5%.
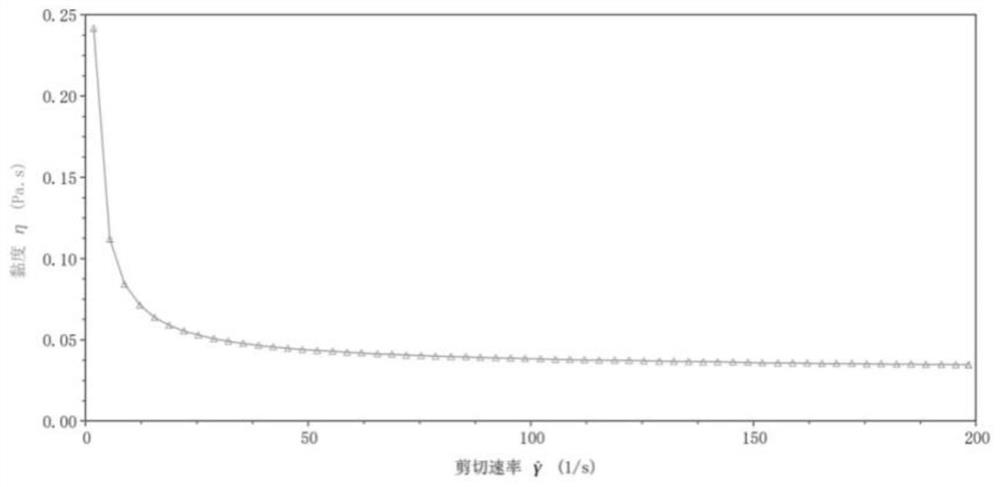
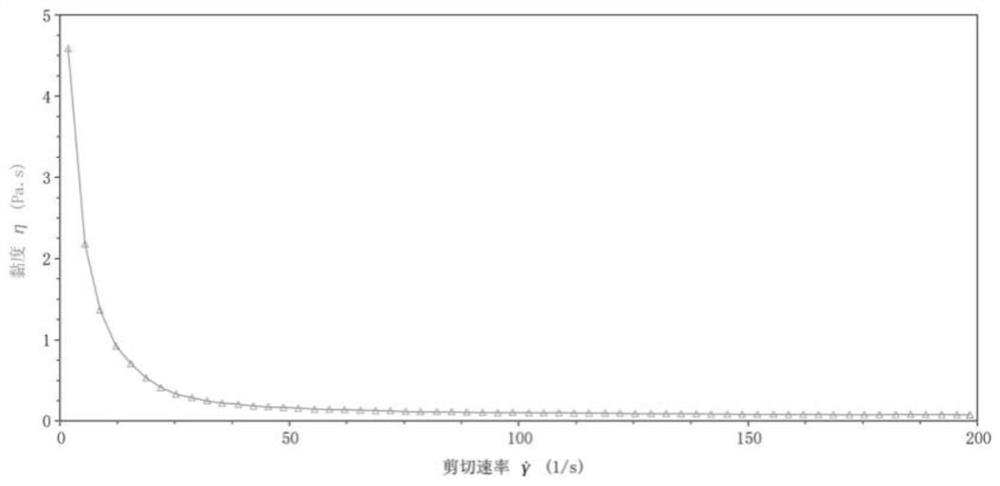
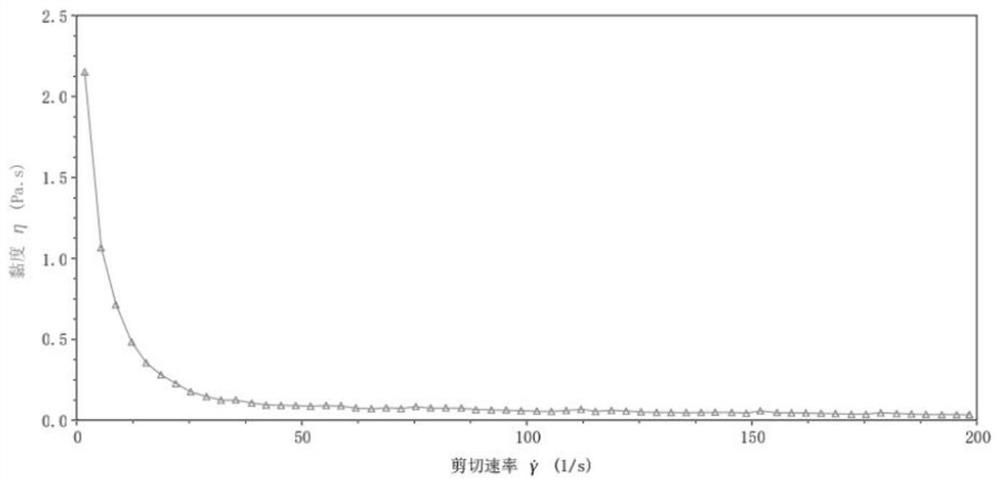
[0094] And by the DHR-1 rheometer, after the semi-cured nutritional agent prepared by this embodiment is brewed, the fluid is in the initial state (pH=7.2), the pH value is 2 and the fluidity of the pH is 4, and the test results are as follows: Figure 1-3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com