Preparation method of o-methylbenzoyl nitrile
A technology of o-toluic acid nitrile and toluic acid nitrile is applied in the field of preparation of o-toluic acid nitrile, and can solve the problems of increasing safety risks, increasing difficulty, and requiring higher safety in production equipment, and the like, Achieving the effect of high yield and chemical purity, simple and feasible preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
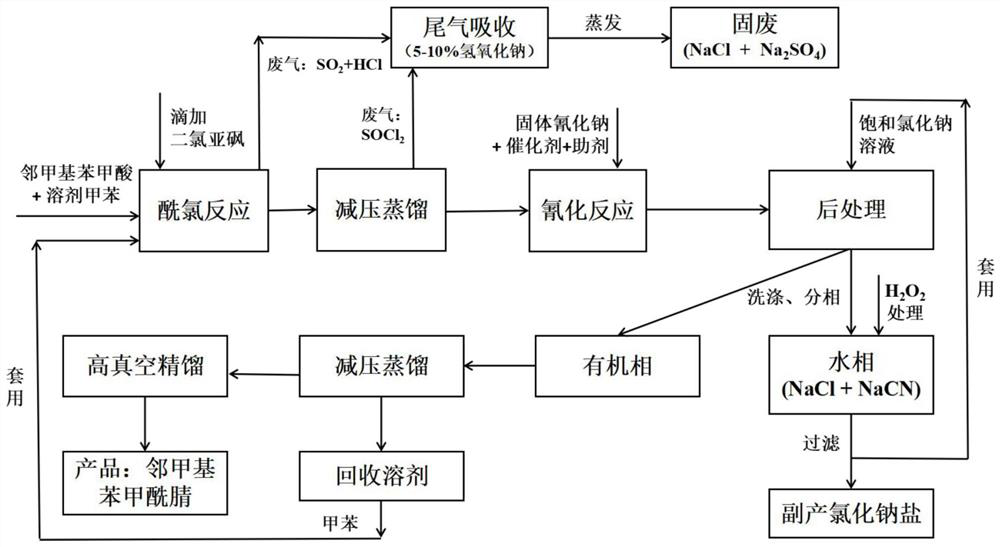
[0043] The embodiment of the present invention discloses a preparation method of o-toluoyl nitrile, specifically comprising the following steps:
[0044]
[0045] (1) o-toluic acid is dissolved in toluene earlier, and thionyl chloride is added dropwise to carry out acyl chloride reaction, after reaction finishes, decompression distillation removes unreacted thionyl chloride, obtains o-toluyl chloride solution, The exhaust gas generated during the process enters the exhaust gas absorption system for treatment;
[0046] Wherein, the mass ratio of o-toluic acid and toluene is 1.0:*(1.0~3.0); the molar ratio of o-toluic acid and thionyl chloride is 1.0:(1.0~2.0); The temperature is 20-60°C; the reaction temperature is 60-80°C, the time is 2-8h, the temperature of vacuum distillation is 40-50°C, the pressure is less than 5kPa, and the tail gas absorption system is 5wt.%-10wt.% hydrogen Sodium oxide solution;
[0047] (2) add catalyzer, solid sodium cyanide and reaction auxilia...
Embodiment 1
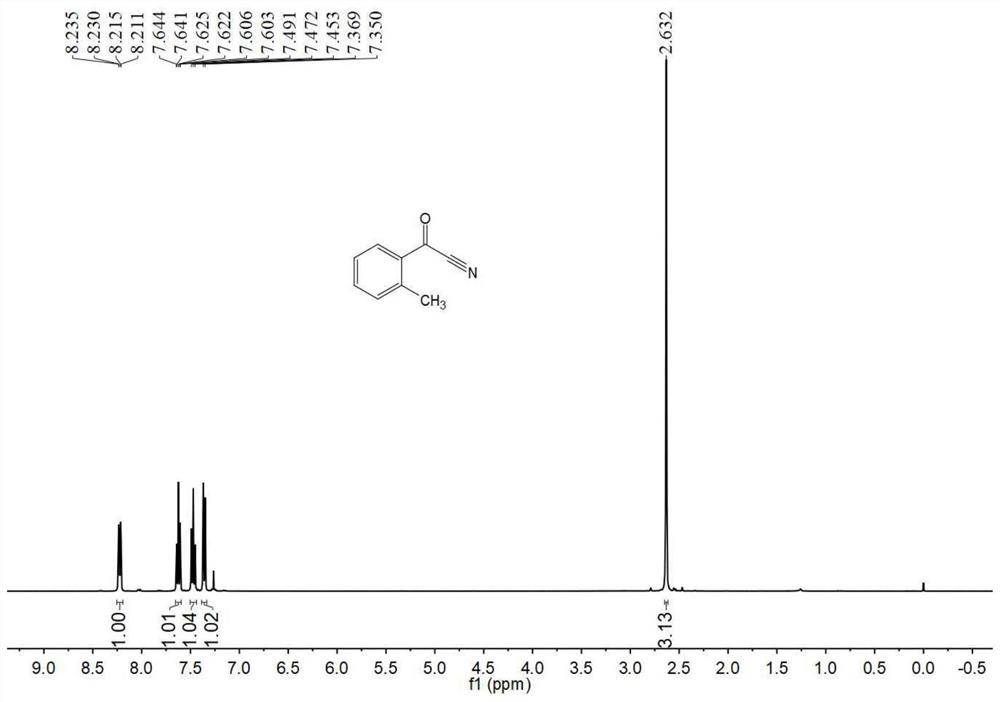
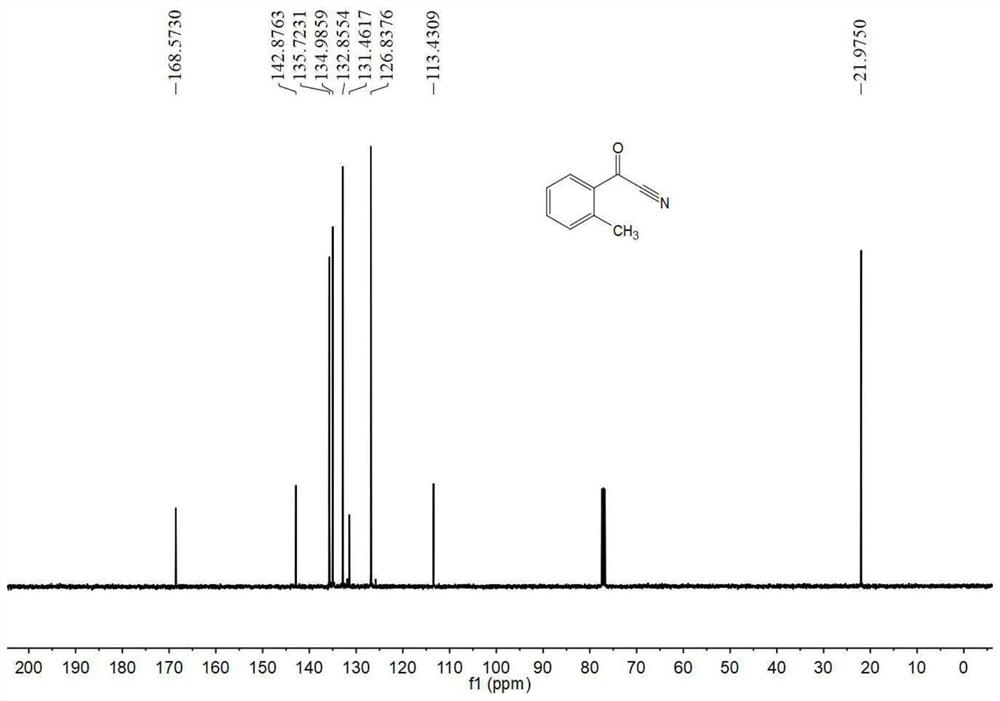
[0055] Embodiment 1 of the present invention discloses a kind of preparation method of o-toluonitrile, specifically comprises the following steps:
[0056] (1) Add 100g (1.0eq) of o-toluic acid and 200g (2.0wt.) of toluene into a 500mL three-necked flask, stir and heat up to 40-45°C, and slowly add 104.86g (1.2eq) of dichloromethylene Sulfone; after the dropwise addition, slowly raise the temperature to 60-65°C, and continue the reaction for 6 hours; after the reaction, cool down to 40-45°C, control the vacuum pump pressure at 2-3kPa, and remove unreacted thionyl chloride by vacuum distillation , to obtain a toluene solution containing the intermediate o-toluyl chloride, and the waste gas produced in the process enters a sodium hydroxide tail gas absorption system with a concentration of 10wt.% for processing;
[0057] (2) Add 1.5g polyethylene glycol (molecular weight 400) and 39.59g (1.1eq) solid sodium cyanide to the toluene solution containing the intermediate o-toluoyl ch...
Embodiment 2
[0061] Embodiment 2 of the present invention discloses a kind of preparation method of o-methylbenzoyl nitrile, specifically comprises the following steps:
[0062] (1) Add 100g (1.0eq) of o-toluic acid and 300g (3.0wt.) of toluene into a 500mL three-necked flask, stir and heat up to 50-55°C, and slowly add 113.6g (1.3eq) of dichloromethylene Sulfone; after the dropwise addition, slowly raise the temperature to 70-75°C, and continue the reaction for 6 hours; after the reaction, cool down to 40-45°C, control the vacuum pump pressure at 2-3kPa, and remove unreacted thionyl chloride by vacuum distillation , to obtain a toluene solution containing the intermediate o-toluyl chloride, and the waste gas produced in the process enters a sodium hydroxide tail gas absorption system with a concentration of 10wt.% for processing;
[0063] (2) Add 3.0g of polyethylene glycol (molecular weight 400) and 41.39g (1.15eq) of solid sodium cyanide to the toluene solution containing the intermedia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com