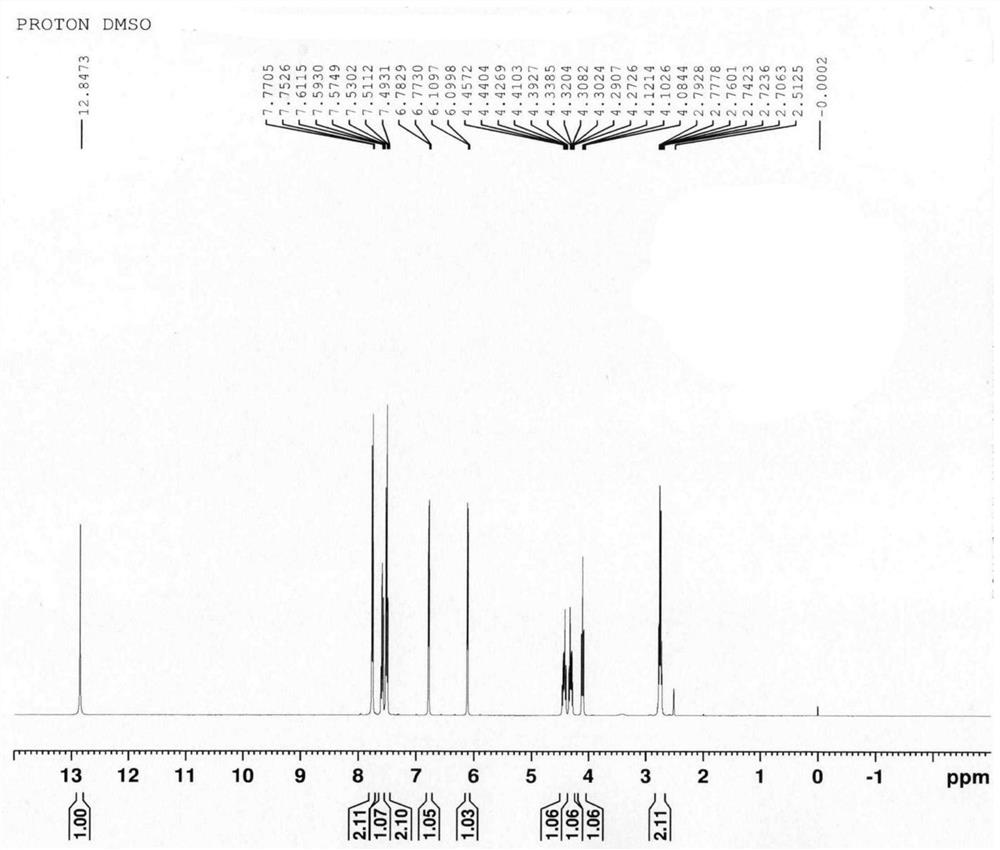
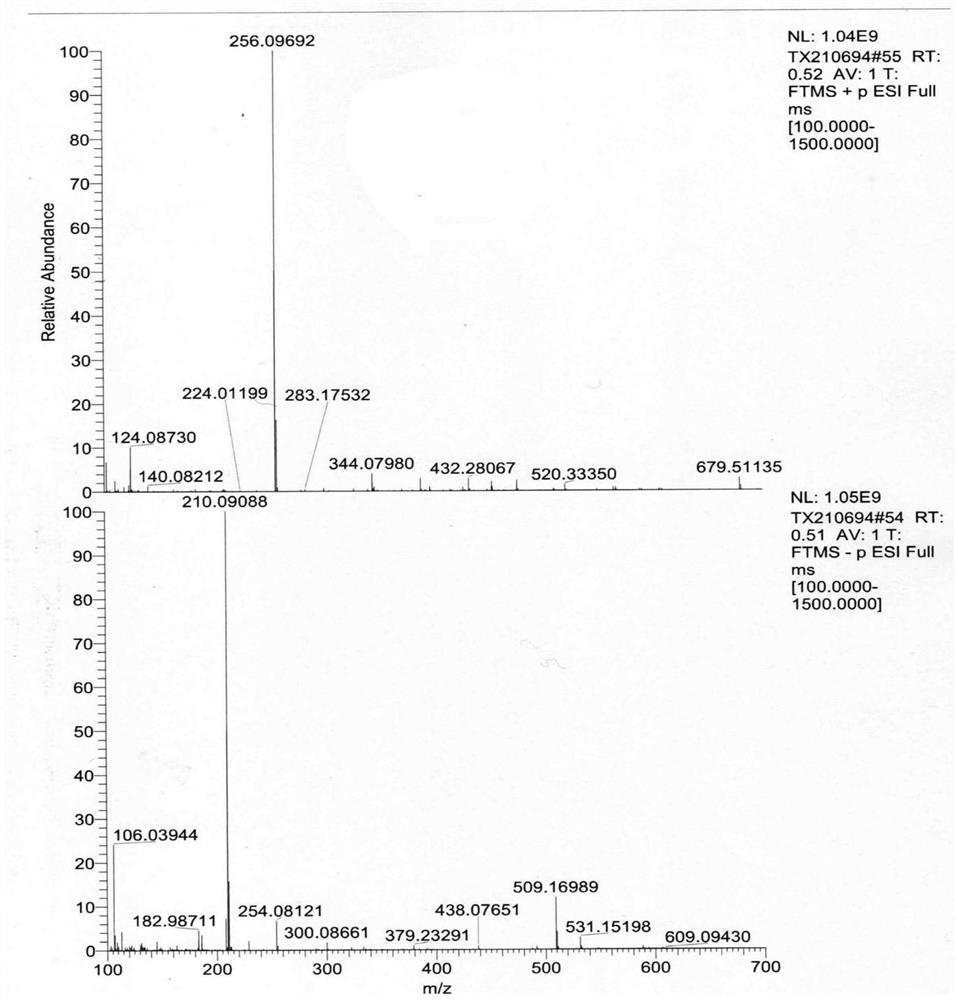
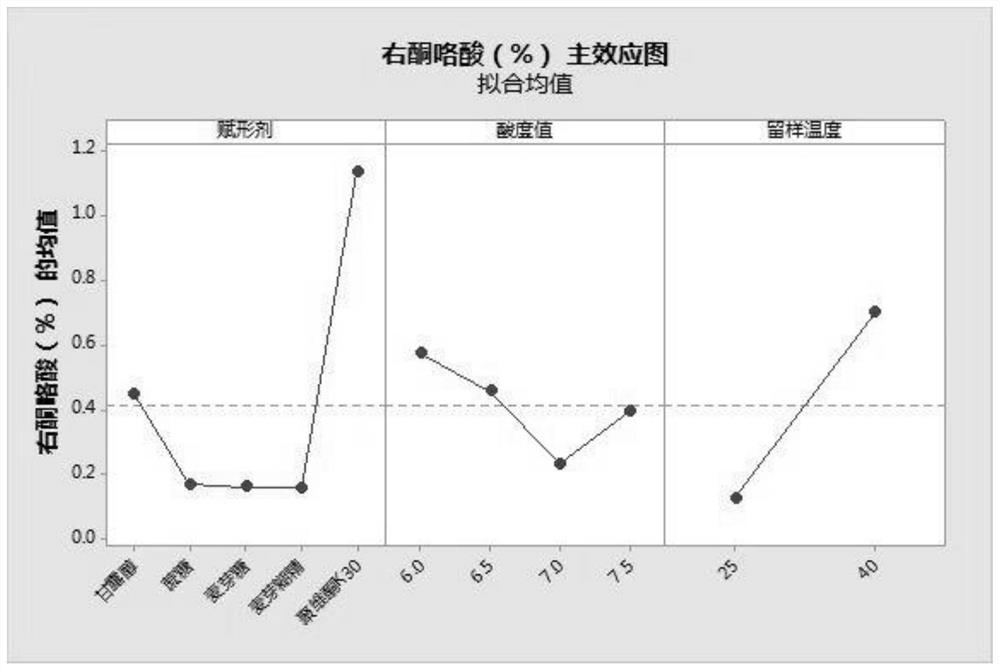
Pharmaceutical composition of levoketorolac and preparation method thereof
A composition, ketorolac technology, applied in the directions of drug combination, drug delivery, pharmaceutical formulation, etc., can solve problems such as blood pressure drop, potential safety hazard, white spot or crystal precipitation, etc., achieve inhibition of three-dimensional transformation, process stability, and operation. handy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0162] The preparation of the freeze-dried powder injection of embodiment 1 levoketorolac sodium salt
[0163] Prescription: Levoketorolac sodium salt: 1.1g; Mannitol: 11g; Anhydrous disodium hydrogen phosphate: 1.44g; 5% sodium dihydrogen phosphate solution: appropriate amount, adjusted to pH6.8; Water for injection: make up to the total amount 200g.
[0164] Preparation:
[0165] Step 1: According to the above prescription, dissolve mannitol and anhydrous disodium hydrogen phosphate with 70-80% water for injection to obtain a clear solution, then add levoketorolac sodium salt and stir to dissolve it, and then dissolve it with 5% dihydrogen phosphate Adjust the pH to 6.8 with sodium solution; make up to the total volume with water for injection;
[0166] Step 2): Sterile filter the drug solution through polyethersulfone filter elements with pore sizes of 0.45 μm, 0.22 μm, and 0.22 μm in sequence, and carry out quality control on the content of the intermediate product solut...
Embodiment 2
[0168] Example 2 Preparation of Levoketorolac Tromethamine Salt Freeze-Dried Powder Injection
[0169] Prescription: Levoketorolac tromethamine salt: 1.5g; Mannitol: 10g; Sodium dihydrogen phosphate dodecahydrate: 1.44g; 0.5mol / L sodium dihydrogen phosphate solution: appropriate amount, adjusted to pH 6.5; Water for injection: make up to a total of 200g.
[0170] Preparation:
[0171] Step 1: According to the above prescription, dissolve mannitol and sodium dihydrogen phosphate dodecahydrate with 70-80% water for injection to obtain a clear solution, then add levoketorolac tromethamine salt and stir to dissolve it, and use 0.5mol / L sodium dihydrogen phosphate solution to adjust the pH to 6.5; make up to the total volume with water for injection;
[0172] Step 2): Sterile filter the drug solution through polyethersulfone filter elements with pore sizes of 0.45 μm, 0.22 μm, and 0.22 μm in sequence, and carry out quality control on the content of the intermediate product soluti...
Embodiment 3
[0174] Example 3 Preparation of Levoketorolac Arginine Salt Freeze-Dried Powder Injection
[0175] Prescription: Levoketorolac arginine salt: 1.7g; Mannitol: 14g; Sodium citrate: 1.5g; 0.5mol / L citric acid solution: appropriate amount, adjust to pH7.0; Water for injection: make up to 200g in total .
[0176] Preparation:
[0177] Step 1: According to the above prescription, dissolve mannitol and sodium citrate with 70-80% water for injection to obtain a clear solution, then add levoketorolac arginine salt and stir to dissolve it, and then add 0.5mol / L citric acid Adjust the pH of the solution to 7.0; make up to the total volume with water for injection;
[0178] Step 2): Sterile filter the drug solution through polyethersulfone filter elements with pore sizes of 0.45 μm, 0.22 μm, and 0.22 μm in sequence, and carry out quality control on the content of the intermediate product solution; divide the drug solution into neutral borosilicate glass tubes In injection vials; after ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific rotation | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com