Porous graphene loaded ferrocobalt sulfide catalyst as well as preparation method and application thereof
A porous graphene and sulfide technology, which is applied in the field of clean energy nanomaterials and catalysis, can solve the problems of easy damage to the porous network structure, complex template etching process, weak contact between heterogeneous interfaces, etc., to make up for low specific surface area. And the effect of insufficient exposure of active sites, accelerated catalytic reaction rate, and uniform particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The mixture hydrogel was prepared by hydrothermal method.
[0041] Concrete preparation steps are as follows:
[0042] 1) Put 10mg / mL graphene oxide water-soluble dispersion and 0.56mg / mL melamine formaldehyde dispersion into the reaction kettle liner according to the ratio of graphene oxide and melamine mass ratio of 1:1.5 and stir rapidly for 2.0 h time.
[0043] 2) 1.0 mL of cobalt metal compound (CoCl 2 ·6H 2 O) precursor aqueous solution and 0.5 mL molar concentration of 0.1 mol / L iron metal compound (FeCl 3 ·6H 2 O) Add the aqueous precursor solution to the mixed dispersion in 1), that is, the molar ratio of the two metal compounds is 1:0.5, stir for 2.0 hours, mix evenly, and take out the magneton.
[0044] 3) Seal the reaction kettle, put it into a constant temperature oven, and keep it warm at 180° C. for 12 hours to obtain the mixture hydrogel.
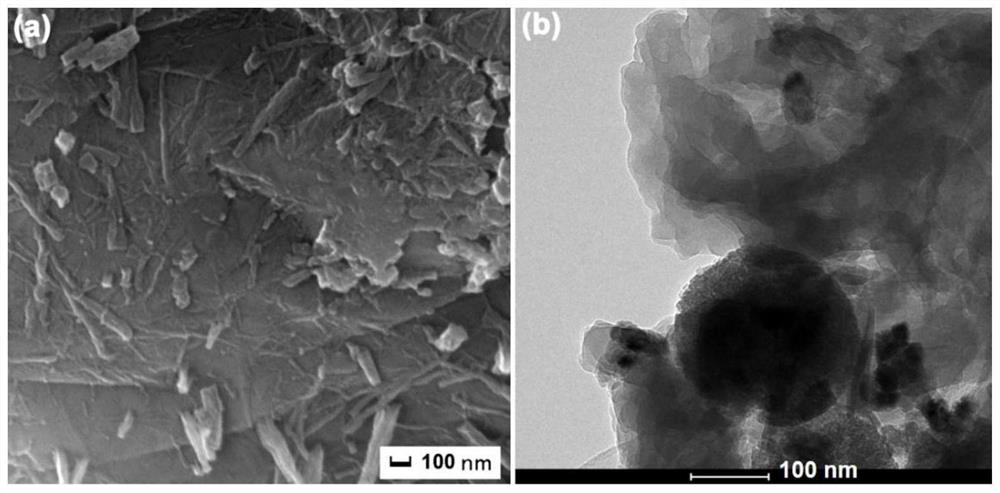
[0045] The morphology of the obtained hybrid hydrogels was characterized by scanning electron microscopy (SEM...
Embodiment 2
[0048] The step of embodiment 2 is similar to that in embodiment 1, other reaction conditions are constant, just the hydrogel obtained in embodiment 1 is mixed with dibenzyl disulfide uniformly, after vacuum drying, carry out heat treatment under argon protection atmosphere, The obtained samples were labeled as Co 8 Fe 8 S 8 @NSG-BA(BA denotes before acid leaching).
[0049] Concrete preparation steps are as follows:
[0050] 1) Redisperse all the mixture hydrogels (10 mL in volume) obtained in Example 1 in a beaker filled with 40 mL of deionized water, add 0.5 g of dibenzyl disulfide, and stir rapidly for 2.0 h; then The beaker was placed in a constant temperature oven at 80°C to dry, and the mixture xerogel was obtained after the water was completely evaporated.
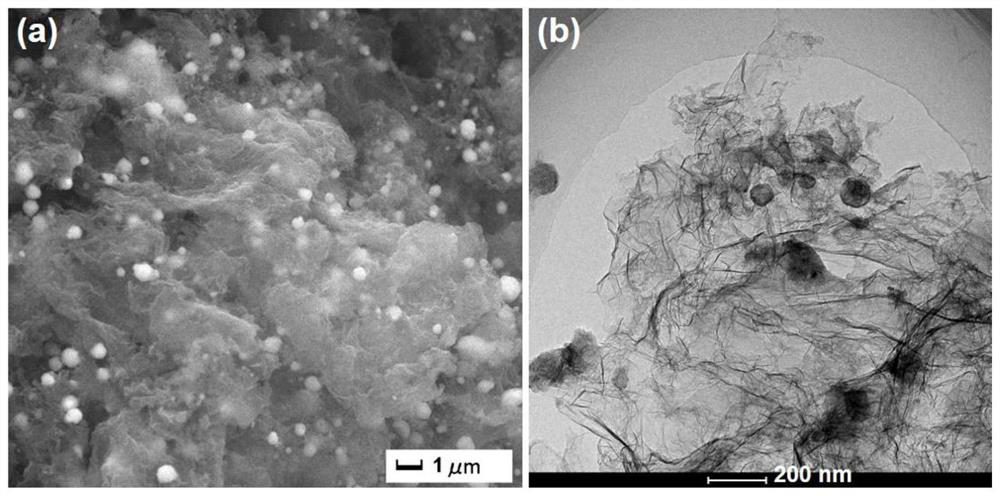
[0051] 2) Grind the xerogel of the mixture obtained in the above 1) and put it into a magnetic boat, transfer it to a tube furnace and heat-treat it at 850°C for 5.0h under an argon atmosphere to obtain a porou...
Embodiment 3
[0055] The steps of embodiment 3 are similar to those in embodiment 2, and other reaction conditions are constant, but the porous graphene-supported cobalt iron sulfide powder material obtained in embodiment 2 is subjected to acid treatment, and the obtained sample is marked as Co 8 Fe 8 S 8 @NSG - A (Adenotes acid leaching).
[0056] Concrete preparation steps are as follows:
[0057] 1) the porous graphene-supported cobalt iron sulfide powder material obtained in embodiment 2 is dispersed in 0.5mol / L H 2 SO 4 In the aqueous solution, 50mL of acid solution was used as the standard according to 1.0g of catalyst, and the sample was stirred and acid-washed for 9.0h at a temperature of 85°C in a condensing reflux device.
[0058] 2) The mixture obtained in the above step 1) is centrifuged and washed with water, and dried to obtain an acid-treated porous graphene-supported cobalt iron sulfide powder sample.
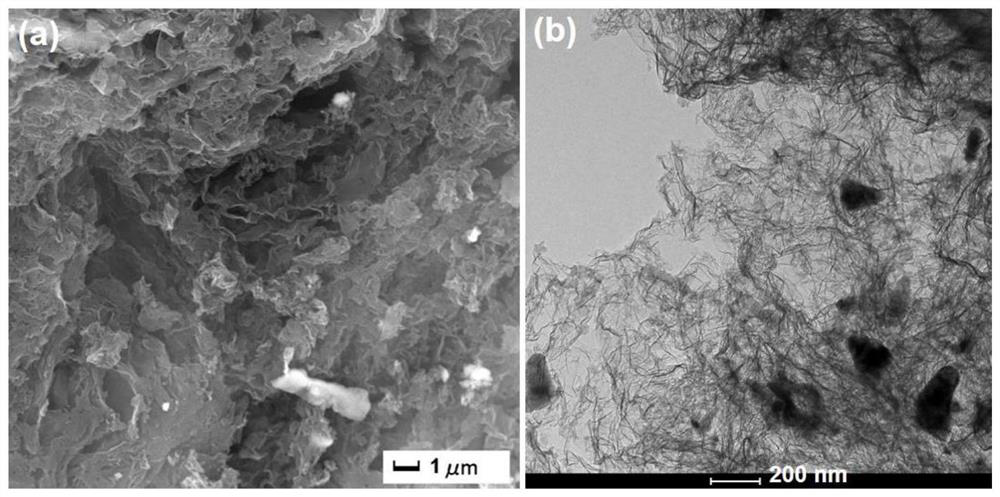
[0059] image 3 It is the SEM and TEM photo of the acid-treated po...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com