Preparation method of trimebutine
The technology of a compound, phenylbutanol, is applied in the preparation of organic compounds, chemical instruments and methods, and the preparation of aminohydroxyl compounds. It can solve the problems of many impurities, affecting product quality and yield, and not easy to operate. Less pollution, high product yield and purity, and the effect of avoiding ether impurities and double bond olefin impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
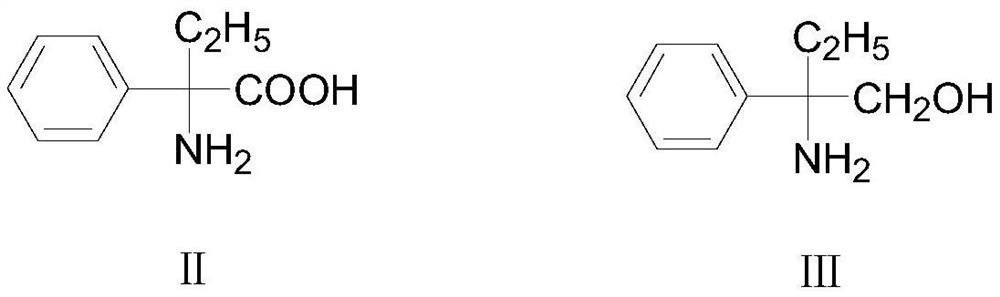
[0031] Preparation of formula Ⅲ compound 2-amino-2-phenylbutanol
[0032]
[0033] Put 134.3g of solvent tetrahydrofuran into a clean four-necked flask, start stirring, and then put in 17.9g (0.1mol) of raw materials 2-amino-2-phenylbutyric acid and 8.3g (0.22mol) of sodium borohydride, and then stir Control the temperature of the system at 10-20°C in the state, and slowly add 12.7g (0.13mol) of sulfuric acid dropwise. After the dropwise addition, keep it warm at room temperature for 12 hours. , the system temperature of the reaction solution was lowered to 10°C, and then 53.7g (0.27mol) of sodium hydroxide aqueous solution with a mass percentage of 20% was added dropwise, and the dropping temperature was controlled at 10-15°C. After the addition was completed, the temperature was raised to reflux for 2h , stop heating and let stand to separate layers, collect the upper organic phase, and concentrate the collected organic phase under reduced pressure to recover tetrahydrofu...
Embodiment 2
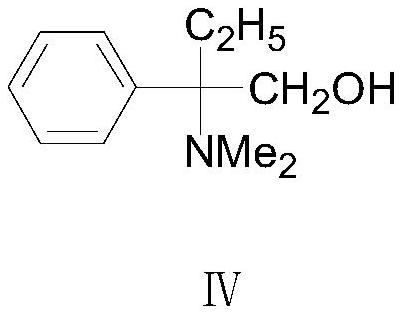
[0035] Preparation of formula Ⅳ compound 2-amino-2-phenylbutanol
[0036]
[0037] Get above-mentioned formula III compound 2-amino-2-phenylbutanol 14.5g (0.086mol) that adopts embodiment 1 to obtain, massfraction is that 85% formic acid 11.6g (0.22mol) and massfraction are 37% formaldehyde 20.9g g (0.26mol) is put into a clean four-necked flask in turn, and the temperature is raised to reflux for 6 hours. During the reaction, the central control can be sampled. The pH value of the system is 10-11, and then extracted twice with toluene (20g×2), the toluene layers obtained by the two extractions are combined, and the toluene layer is washed twice with water (15g×2), and the toluene layer obtained is carried out The solvent was distilled off under reduced pressure to obtain 15.8 g of the corresponding intermediate compound of formula IV, 2-amino-2-phenylbutanol, as a brown viscous liquid with a GC content of 95% and a yield of 95%.
Embodiment 3
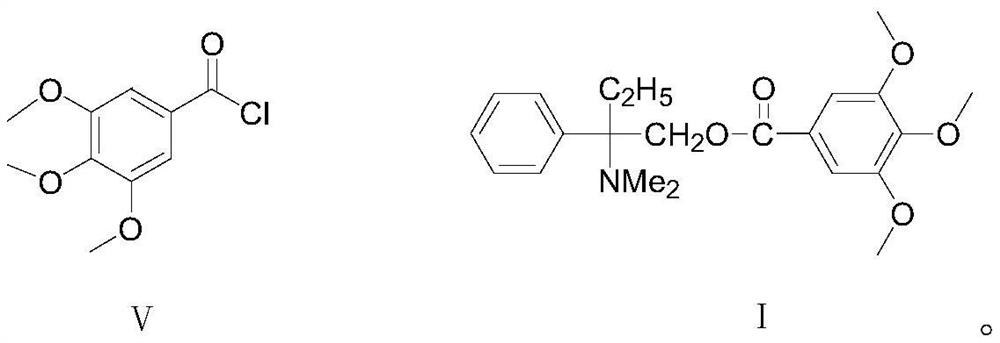
[0039] The preparation of formula I compound trimebutine
[0040]
[0041] Add water 31.6g, sodium bicarbonate 9.61g (0.12mol), acetone 50g and formula IV compound 2-amino-2-phenylbutanol 15.8g (0.082mol) successively in a clean four-necked reaction flask, and cool down the system After reaching 5°C to 10°C, slowly add dropwise a mixture of 22.62g (0.098mol) of the compound of formula V and 29g of acetone. During the dropwise addition, control the temperature at 5°C to 10°C. Carry out insulation reaction 2h, after reaction finishes, carry out decompression distillation and reclaim acetone, filter, the wet product solid product that obtains is rinsed with water twice (15g * 2), dry, obtain final product trimebutine 29.14g, off-white To light yellow crystalline powder, the HPLC purity is 99.5%, the single impurity is <0.1%, and the yield is 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com