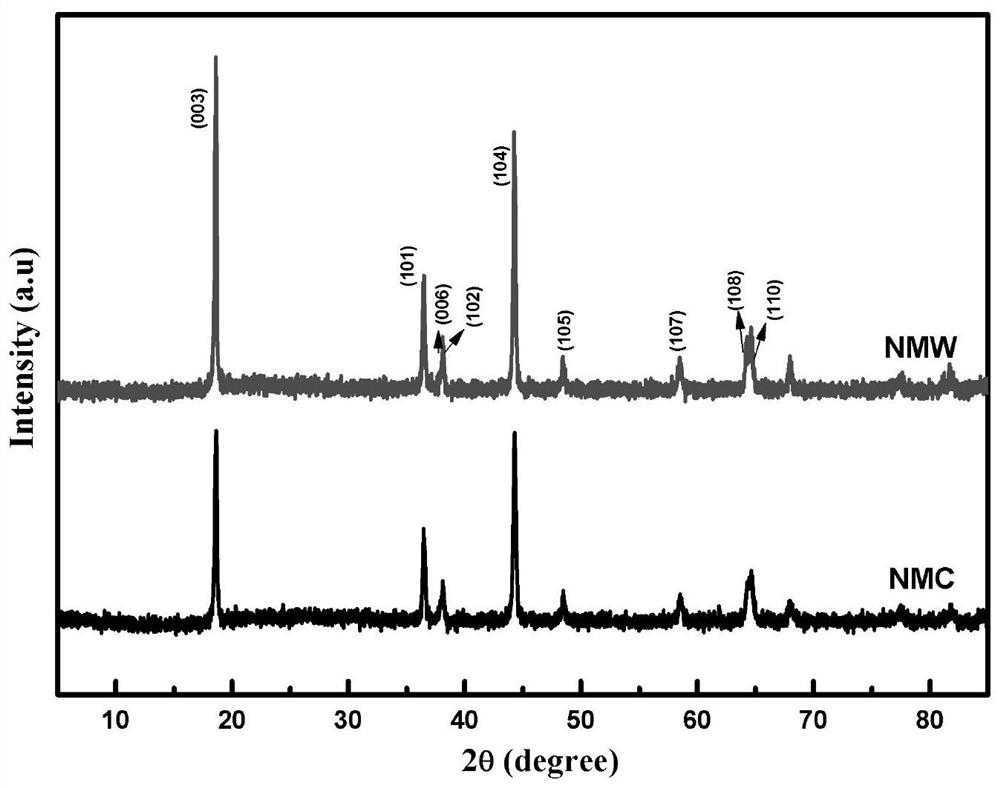
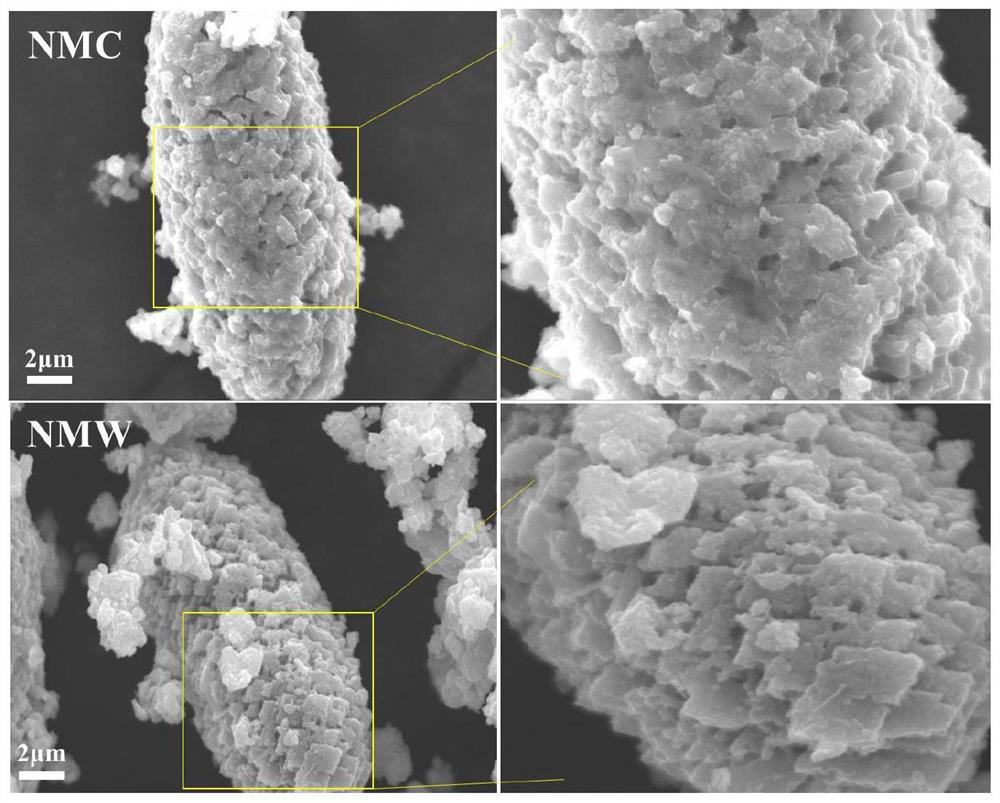
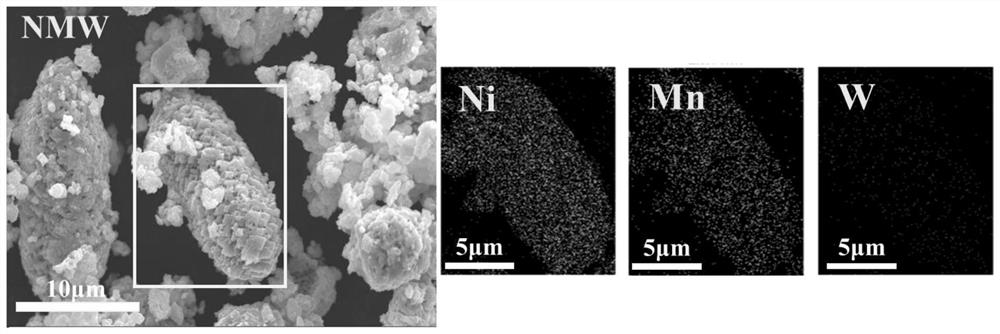
Nickel-manganese-tungsten lithium ion battery positive electrode material and preparation method thereof
A technology for lithium-ion batteries and positive electrode materials, applied in the field of nickel-manganese-tungsten lithium-ion battery positive electrode materials and its preparation, can solve problems such as unsatisfactory electrochemical performance, reduced material capacity retention rate and thermal stability, and unstable crystal structure. , to achieve the effect of shortening the transmission path of lithium ions, facilitating engineering applications, and uniform distribution of shape and size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] A preparation method of nickel-manganese-tungsten lithium-ion battery cathode material, comprising the following steps:
[0044] (1) Take nickel acetate, manganese acetate, tungsten acetate according to the mol ratio of Ni:Mn:W=0.85:0.14:0.01, mix and dissolve with deionized water, form solution A after stirring; ): urea=1:3.5 molar ratio weighed urea, dissolved with deionized water and stirred evenly to form solution B; solution B was gradually dropped into solution A to form a mixed reaction solution;
[0045] (2) Pour the mixed reaction liquid into the liner of the reaction kettle, place it in an oven and keep it warm at 180°C for 8 hours, and obtain the reaction precipitation liquid after solvothermal reaction;
[0046] (3) After the reaction precipitation solution is cooled, wash it with deionized water through a centrifuge at a speed of 3000r / min. After repeated washing and reaction, the precipitation solution is dried at 80°C in a blast drying oven to obtain nick...
Embodiment 2
[0050] A preparation method of nickel-manganese-tungsten lithium-ion battery cathode material, comprising the following steps:
[0051] (1) Take nickel sulfate, manganese sulfate, tungsten oxide according to the mol ratio of Ni:Mn:W=0.90:0.03:0.07, mix and dissolve with deionized water, form solution A after stirring; ): Ammonium bicarbonate=1:2.5 molar ratio Weigh ammonium bicarbonate, dissolve and stir with deionized water to form solution B, gradually drop solution B into solution A to form a mixed reaction solution;
[0052] (2) Pour the mixed reaction liquid into the liner of the reaction kettle, place it in an oven and keep it warm at 150°C for 10 hours, and obtain the reaction precipitation liquid after the solvothermal reaction;
[0053] (3) After the reaction precipitation solution is cooled, wash it with deionized water through a centrifuge at a speed of 5000r / min. After repeated washing and reaction, the precipitation solution is dried at 60°C in a blast drying oven...
Embodiment 3
[0057] A preparation method of nickel-manganese-tungsten lithium-ion battery cathode material, comprising the following steps:
[0058] (1) Weigh nickel acetate, manganese acetate, ammonium tungstate according to the molar ratio of Ni:Mn:W=0.88:0.10:0.02, mix and dissolve with deionized water, form solution A after stirring evenly, then (Ni+Mn +W): oxalic acid=1:1 molar ratio Weigh oxalic acid, dissolve it with deionized water and stir evenly to form solution B, gradually drop solution B into solution A to form a mixed reaction solution;
[0059] (2) Pour the mixed reaction liquid into the liner of the reaction kettle, place it in an oven and keep it warm at 200°C for 5 hours, and obtain the reaction precipitation liquid after the solvothermal reaction;
[0060] (3) After the reaction precipitation solution is cooled, wash it with deionized water through a centrifuge at a speed of 8000r / min, and the precipitation solution after repeated washing and reaction is dried at 100°C i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| retention rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com