Diaphragm electrolysis method for preparing carbon monoxide and pypocholoride in micro-gap electrolytic tank
A technology of hypochlorite and carbon monoxide, applied in the direction of electrolysis components, electrolysis process, etc., can solve the problems of low cathode reaction current density, accelerated hydrogen evolution reaction speed, no economic feasibility, etc., and achieves easy start and stop and low production cost. , The effect of the equipment occupying a large area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
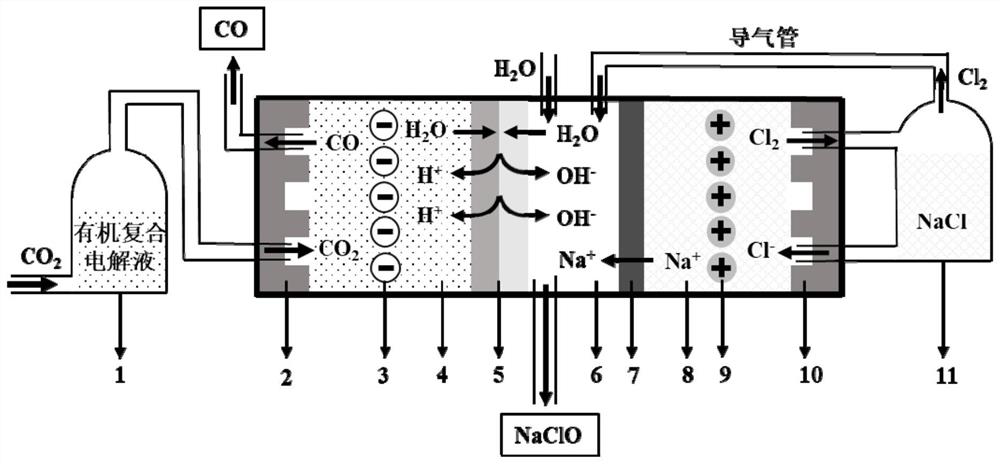
[0041] The diaphragm electrolysis method for preparing carbon monoxide and hypochlorite in a micro-gap electrolytic cell, the specific operation steps are as follows:
[0042] Step 1: The electrolytic cell is divided into a cathode compartment, an intermediate compartment and an anode compartment by a bipolar membrane and a perfluorosulfonic acid type cation exchange membrane. A porous Au electrode is placed in the cathode compartment as a cathode, and a porous iridium oxide coating is placed in the anode compartment. A layered titanium electrode was used as the anode, and water was added to the intermediate chamber. The anion permeation layer of the bipolar membrane is an imidazole polyether ether ketone anion permeation layer with a thickness of 200 microns, and the cation permeation layer is a perfluorosulfonic acid type cation permeation layer with a thickness of 150 microns. The interfacial region of the layer introduces titanium oxide / nickel oxide nanoparticles as a wate...
Embodiment 2
[0047] The diaphragm electrolysis method for preparing carbon monoxide and hypochlorite in a micro-gap electrolytic cell, the specific operation steps are as follows:
[0048] Step 1: The electrolytic cell is divided into a cathode compartment, an intermediate compartment and an anode compartment by a bipolar membrane and a sulfonated polyethylene cation exchange membrane. A porous Ag electrode is placed in the cathode compartment as a cathode, and a porous IrO is placed in the anode compartment. 2 ·Ta 2 O 5 The coated titanium electrode acts as the anode, and water is added to the intermediate chamber. The anion permeation layer of the bipolar membrane is a sub-permeation layer of a diamine-containing styrene / vinylbenzyl chloride copolymer anion permeation layer with a thickness of 180 microns, and the cation permeation layer is a sulfonated polyethylene cation permeation layer with a thickness of 180 microns. 250 microns, and polyvinyl acid / polyvinyl pyridinium complex was...
Embodiment 3
[0053] The diaphragm electrolysis method for preparing carbon monoxide and hypochlorite in a micro-gap electrolytic cell, the specific operation steps are as follows:
[0054] Step 1. The electrolytic cell is divided into a cathode compartment, an intermediate compartment and an anode compartment by a bipolar membrane and a sulfonated polystyrene cation exchange membrane. A porous Zn electrode is placed in the cathode compartment as a cathode, and a porous glassy carbon electrode is placed in the anode compartment. As an anode, water is added to the intermediate chamber. The anion permeation layer of the bipolar membrane is a quaternized polyvinyl chloride anion permeation layer with a thickness of 210 microns, and the cation permeation layer is a sulfonated polyvinylidene fluoride cation permeation layer with a thickness of 150 microns. The interfacial region of the permeable layer introduced sulfonated polyetheretherketone as a water dissociation catalyst.
[0055] Step 2, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com