Full-bio-based aryl diamine chain extender as well as preparation method and application thereof
A base aryl diamine chain extender, base aryl diamine technology, applied in the field of all bio-based aryl diamine chain extenders and its preparation, can solve the problem of structure containing halogen, molecular structure easily absorbed by human body, carcinogenic Effect and other issues, to achieve the effect of improving tensile strength, avoiding dependence on fossil resources, and high reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-5
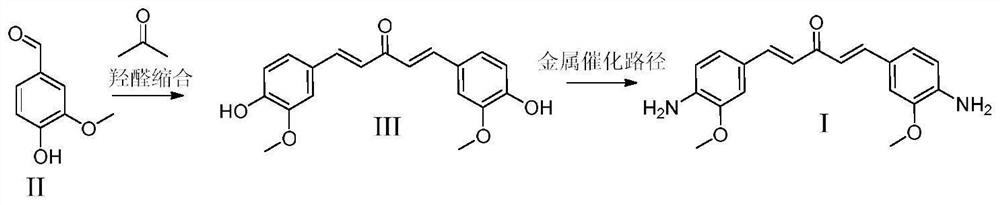
[0084] To the reaction bottle was added p-hydroxybenzaldehyde (20mmol, 2.44g), acetone (10mmol, 0.58g), the solvent was methanol 50mL, and the catalyst 1mmol was DBU, TBD, NaOH, CaO and alkaline ionic liquids, respectively + [NH 3 CH 2 CH 2 OH][CH 3 COO] - The reaction was reacted at 70 °C for 3h, the reaction liquid was taken for HPLC detection, the conversion rate and yield were shown in Table 2 below, the reaction liquid was filtered or directly concentrated after the reaction was completed, water and ethyl acetate were added for extraction, and the organic phase was separated for column chromatography purification (ethyl acetate / n-hexane = 1:1 gradient elution), that is, compound III1 was obtained. 1 H NMR(400MHz,CDCl 3 )7.70(d,J=16Hz,2H),7.57(dd,J=6.7,2Hz,4H),7.05(d,J=16Hz,2H),6.83(dd,J=6.7,2Hz,4H); 13 C NMR(100MHz,CDCl 3 )δ115.8,123.3,127.8,130.6,142.2,157.7,188.6.MSI-MS:289.3[M+Na] + 。
[0085] Table 2 Example 1-5 Catalytic reaction efficiency of different catalysts
[00...
Embodiment 6
[0088]Metal-free catalyzed phenol hydroxylamine reaction process: weigh III1 (10mmol, 2.66g), chloroacetylamide (20mmol, 1.87g), anhydrous potassium carbonate (25mmol, 3.45g), potassium iodide (1mmol, 0.27g) in a 1L round bottom flask, add 700mL acetone, stirred at 60 °C for 6h, filtered after the end of the reaction, filtrate dried, water and ethyl acetate extraction, anhydrous magnesium sulfate dried and concentrated organic phase, The recrystallization of chloroacetamide product V1 was obtained, and the yield was 98.2%. 1 H NMR(400MHz,DMSO-d6)δ7.82(d,2H),7.68(dd,4H),7.28(s,4H),7.03(d,2H),6.88(dd,4H),4.64(s,4H). 13 C NMR(100MHz,DMSO-d6)δ74.2,114.2,123.3,127.5,130.2,142.2,157.6,170.5,189.3.MSI-MS:403.3[M+Na] + 。
[0089] Accurately weigh the chloroacetamide product (10mmol, 3.80g), potassium hydroxide (40mmol, 2.24g) in a microwave reaction bottle, add 150mL dimethyl sulfoxide (DMSO) and 50mL N, N-dimethacrylamino (DMPU), microwave heating at 180 °C for 2h, after the reaction end...
Embodiment 7
[0091]Metal-catalyzed phenol hydroxylamine reaction process: accurately weigh compound III1 (0.133g, 0.5mmol), Reney nickel (0.05g, 0.85mmol) and tert-pentanol 25ml in the reactor, into 10 bar ammonia, 170 °C reaction for 18h. After the end of the reaction, the reaction solution was tested by HPLC, and the yield was 96.0%. After the reaction is finished, the filtrate is drained, and the column chromatography (ethyl acetate / n-hexane) is separated and purified.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tensile strength | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com