Synthesis of nano hollow balls of zinc selenide
A synthesis method, zinc selenide technology, applied in chemical instruments and methods, binary selenium/tellurium compounds, zinc compounds, etc., can solve the problem of micron-scale ZnSe hollow spheres that cannot be synthesized, and micron-sized hollow particles that cannot be synthesized with zinc selenide Balls, unable to be further assembled into photonic crystals, etc., to achieve the effects of easy control, cheap raw materials, and stable product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
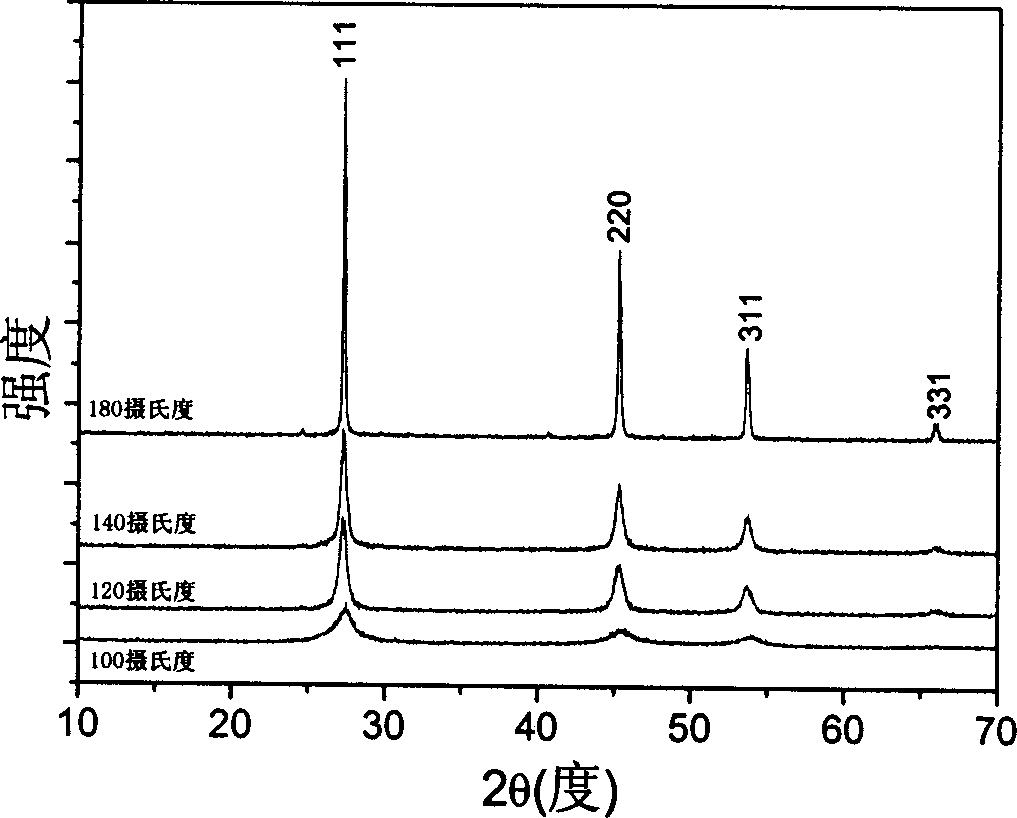
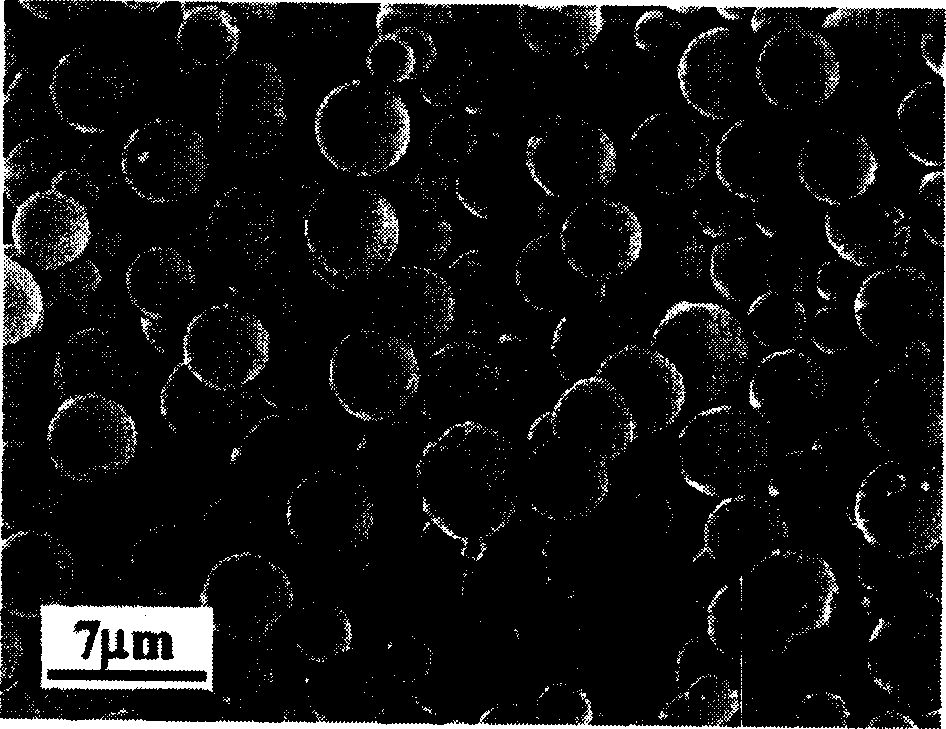
[0049] Weigh 0.002 mole of analytical pure ZnSO 4 ·7H 2 O is placed in a 40 ml reaction kettle, add 20 ml of 1M NaOH solution to dissolve it, add equimolar amount of sodium selenite and 1 times molar excess of hydrazine hydrate into this reaction kettle, add deionized water to the total volume 70%, seal the reactor, and react at 100°C for 2 hours. Then it was cooled to room temperature, the reaction kettle was opened, filtered with a cloth funnel, washed with deionized water, and a yellow powder was obtained. The product was identified as zinc selenide by X-ray powder diffraction, and it was identified as a micron-level zinc selenide ball by scanning electron microscopy, with a diameter of about 3 microns (attached figure 2 ). After grinding the zinc selenide ball, it was identified with a scanning electron microscope, and it was found that the ball has a hollow structure (attached Figure 4 ).
[0050] Under the same conditions, replace hydrazine hydrate with a molar excess of 5...
Embodiment 2
[0052] Weigh 0.003 moles of analytical pure ZnCl 2 Placed in a 40 ml reaction kettle, add 20 ml of 2M NaOH solution to dissolve, and the AgNO 3 Add it to the solution at the ratio of Zn:Ag=100:5, and add it with ZnCl 2 And 1 / 2 times AgNO 3 The sum of moles of ammonium selenite, 2 times excess of hydrazine sulfate, and finally add deionized water to 70% of the total volume, seal the reactor and react at 120°C for 4 hours to prepare a Zn:Ag ratio of 100:5 Ag doped composite zinc selenide micron hollow spheres.
[0053] Under the same conditions, replace hydrazine hydrate with sodium borohydride, potassium borohydride, hydroxylamine or hydrazine sulfate, and control the reaction temperature at 100, 150, 200℃, and adjust the reaction time to 48, 32, 16, 8, 2 hours , Can get micron-sized Ag-doped zinc selenide hollow spheres.
Embodiment 3
[0055] Weigh 0.002 mole analytically pure Zn (NO 3 ) 2 Placed in a 40 ml reaction kettle, add 20 ml of 1M KOH solution to dissolve, and MnSO 4 Add it to the solution at the ratio of Zn:Ni=100:10, and add it with Zn(NO 3 ) 2 And NiSO 4 The sum of the molar amount of selenous acid, the excess of sodium borohydride 4 times, and finally add deionized water to 70% of the total volume, seal the reactor, and react at 160°C for 32 hours to prepare a Zn:Ni ratio of 100:10 Ni-doped composite zinc selenide micron hollow sphere (attached Figure 5 ).
[0056] Under the same conditions, replace hydrazine hydrate with sodium borohydride, potassium borohydride, hydroxylamine or hydrazine sulfate, control the reaction temperature at 100, 140, 200℃, and adjust the reaction time to 48, 32, 16, 8, 4 hours , Can get micron-scale Ni-doped zinc selenide hollow spheres.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com