Biodegradable targetable microparticle delivery system
a delivery system and biodegradable technology, applied in the field of biodegradable microparticles, can solve the problems of many nonliving liquid vaccines, and many antigens that fail to induce protective immune responses or only weak immune responses, and achieve the effect of improving encapsulation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0134] This Example illustrates the preparation of N-Z-L-Serine β-Lactone.
[0135] The preparation of this cyclic N-protected amino acid lactone was based on a modified procedure in which an N-protected-α-amino acid is reacted to yield cyclized pseudo-α-amino acid monomer (ref. 6). All glassware was pre-dried overnight in an oven set at 120° C. Prior to use it was cooled in a vacuum desiccator and purged under a stream of dry nitrogen for 10 minutes.
[0136] To a 1 L three necked round bottomed flask under nitrogen was added triphenylphosphine (TPP; Aldrich; 7.87 mL; 50 mmol; FW: 174.16). To this was added 200 mL of anhydrous acetonitrile (CH3CN; Aldrich): anhydrous tetrahydrofuran (THF; Aldrich) solution (volume ratio 85:15) via syringe and stirred until the solid TPP was dissolved. To this solution diethyl azodicarboxylate (DEAD; Aldrich; 7.87 mL; 50 mmol; FW: 262.29) was added via syringe and the solution stirred at room temperature for 30 minutes. The solution was then cooled to a...
example 2
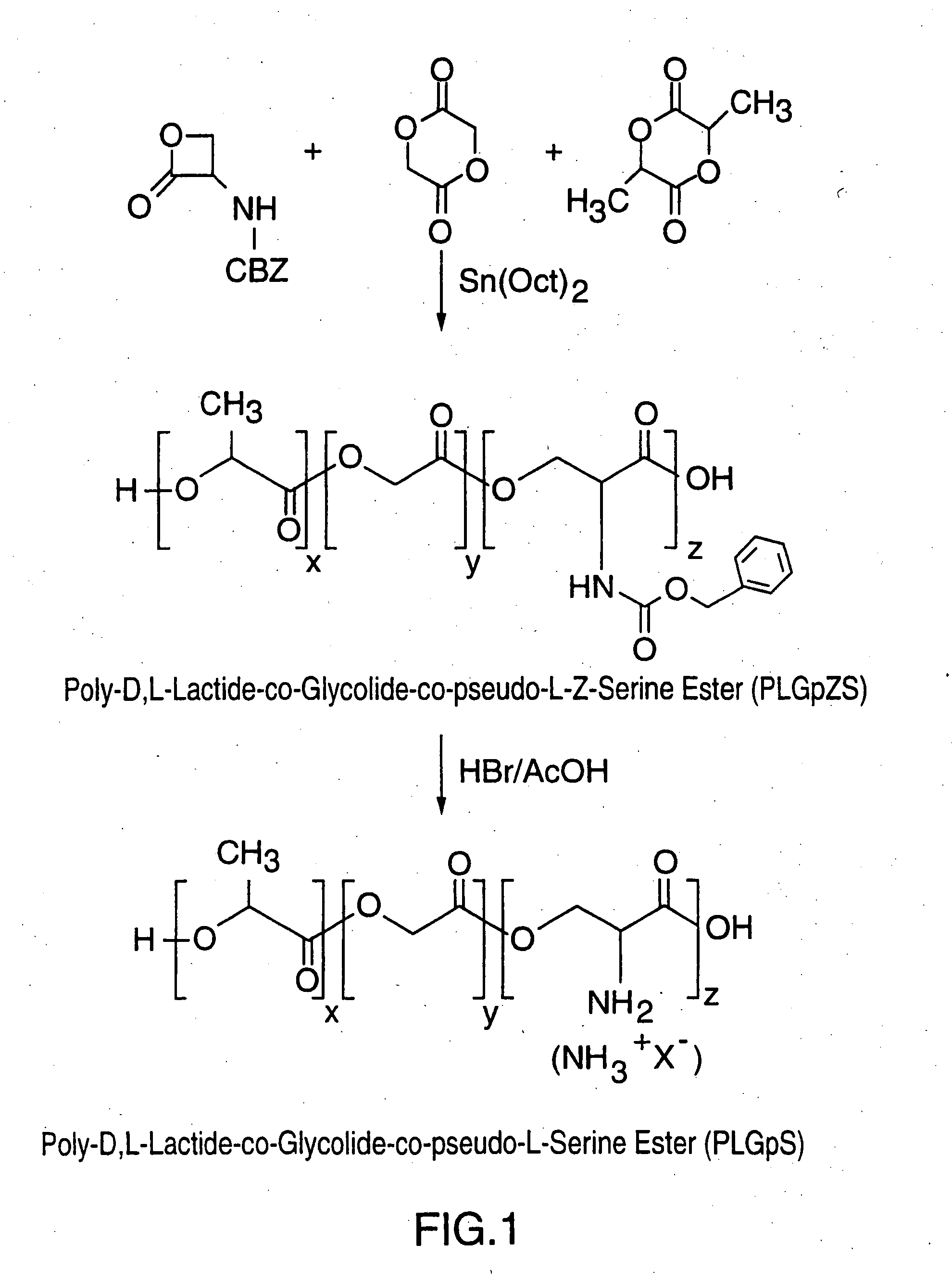
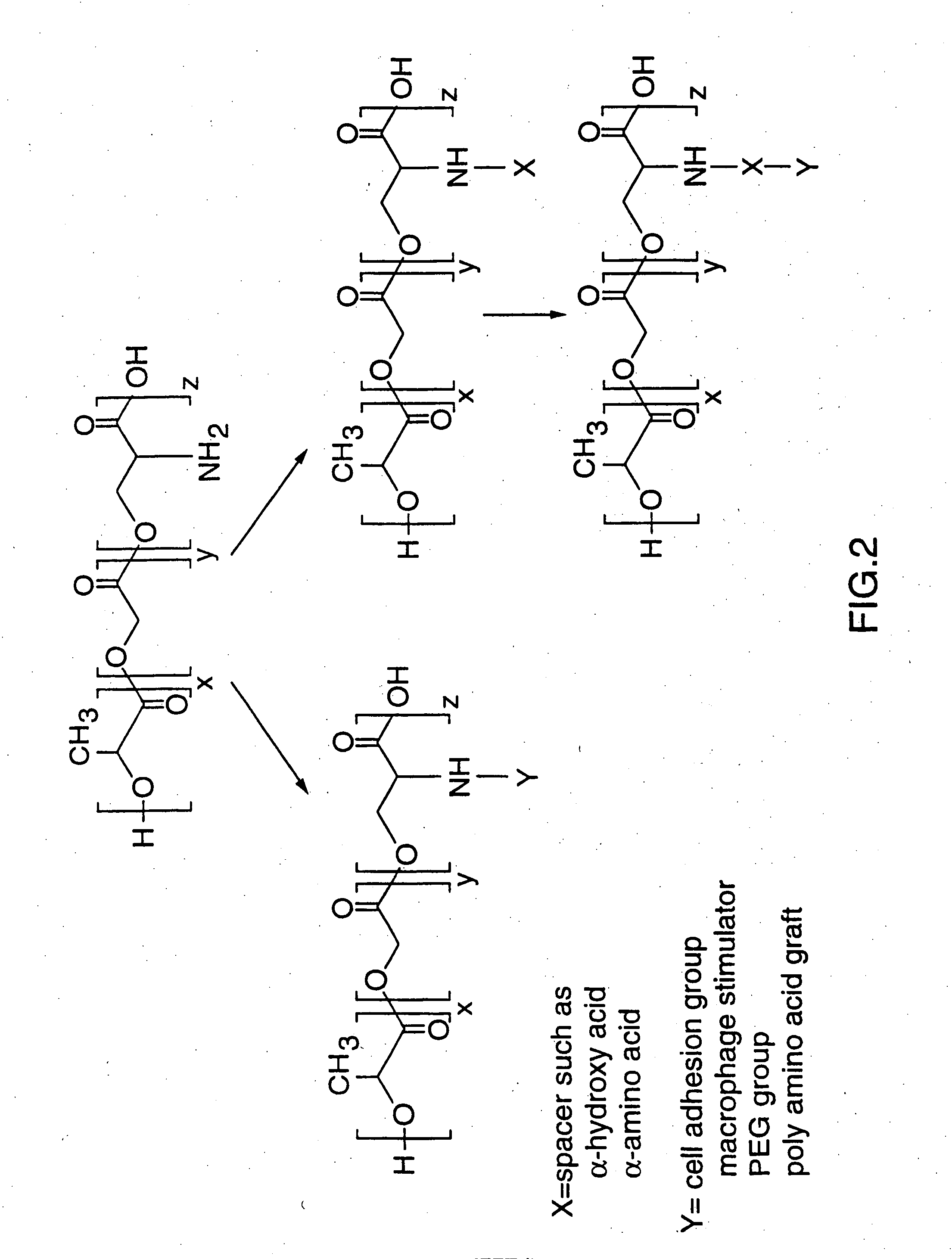
[0138] This Example illustrates the preparation of the copolymer poly-D,L-Lactide-co-Glycolide-co-pseudo-Z-Serine Ester (PLGpZS) as shown in FIG. 1.
[0139] Glassware was pre-dried overnight. Prior to use it was cooled in a vacuum desiccator. Additionally the polymerization vessel (glass ampule) must be siliconized (SurfaSil; Pierce; 2% solution in toluene) and all transfer reactions and additions of reagents and monomers to polymerization vessel must be conducted in a glove box maintained under a dry nitrogen environment.
[0140] Prior to polymerization the D,L-lactide (2,6-dimethyl-1,4-dioxane-2,5-dione; Aldrich; FW: 144.13) and glycolide (Boehringer Ingelheim; FW: 116.096) was recrystallized from anhydrous ethyl acetate in the glove box and dried in vacuo for about 2 days. Once fully dried the monomers can be stored in the glove box with the freshly recrystallized serine lactone (stored at 0° C.) of Example 1 brought directly into the glove box. All monomers and catalyst / initiators...
example 3
[0145] This Example illustrates the preparation of the copolymer poly-D,L-Lactide-co-Glycolide-co-pseudo-Serine Ester (PLGpS) as shown in FIG. 1.
[0146] All glassware was pre-dried overnight. Prior to use it was cooled in a vacuum desiccator and purged under a stream of dry nitrogen for 10 minutes. All reactions were conducted under inert atmosphere of dry nitrogen.
[0147] To a 2 necked 100 mL round bottomed flask equipped with a stir bar was placed 400 mg of polymer (PLGpZS). To this was added a 10 mL solution of 30% hydrogen bromide in acetic acid (Aldrich; FW: 80.92) which was sufficient for slurry formation. The slurry was stirred for 30 to 45 minutes and quenched by dropwise addition of anhydrous diethyl ether (Aldrich) followed by anhydrous methanol (Aldrich). This results in polymer precipitation which was then isolated by vacuum filtration. The crude polymer precipitate was washed with diethyl ether and reprecipitated from chloroform:hexane. The purified polymer was dried in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com