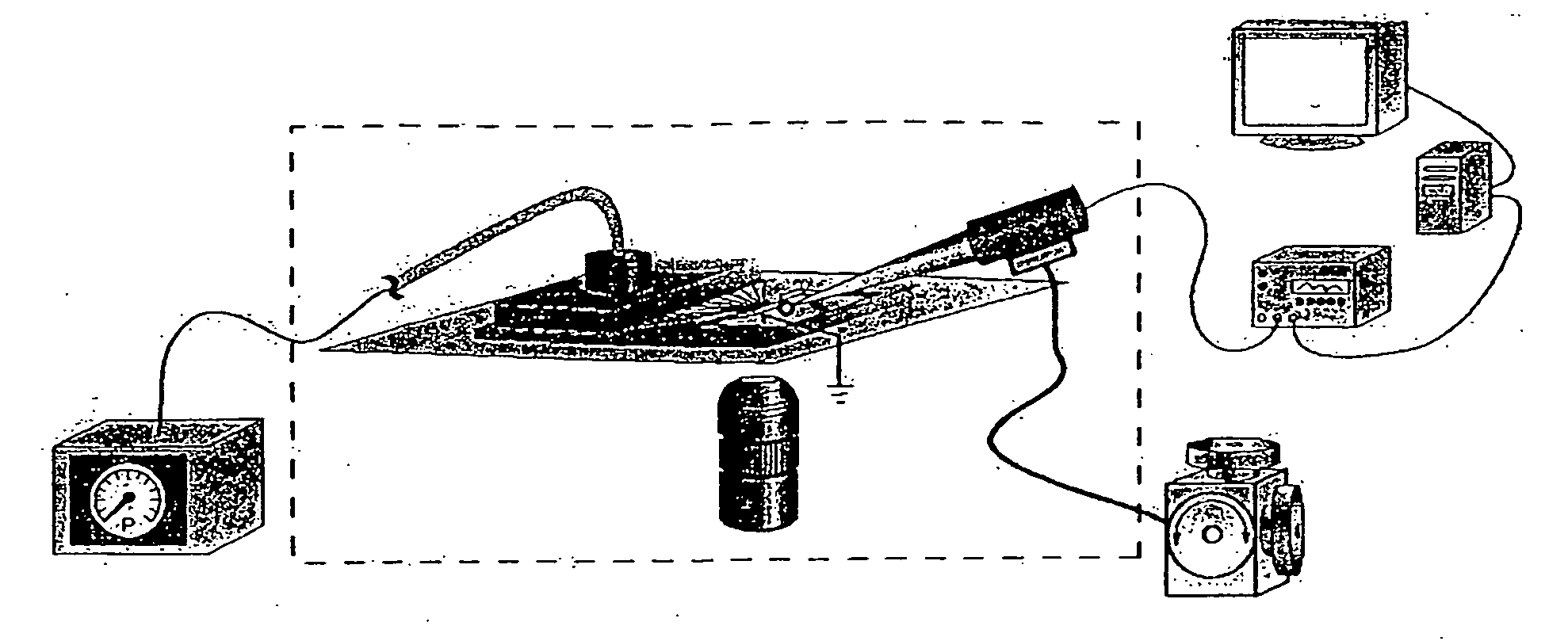
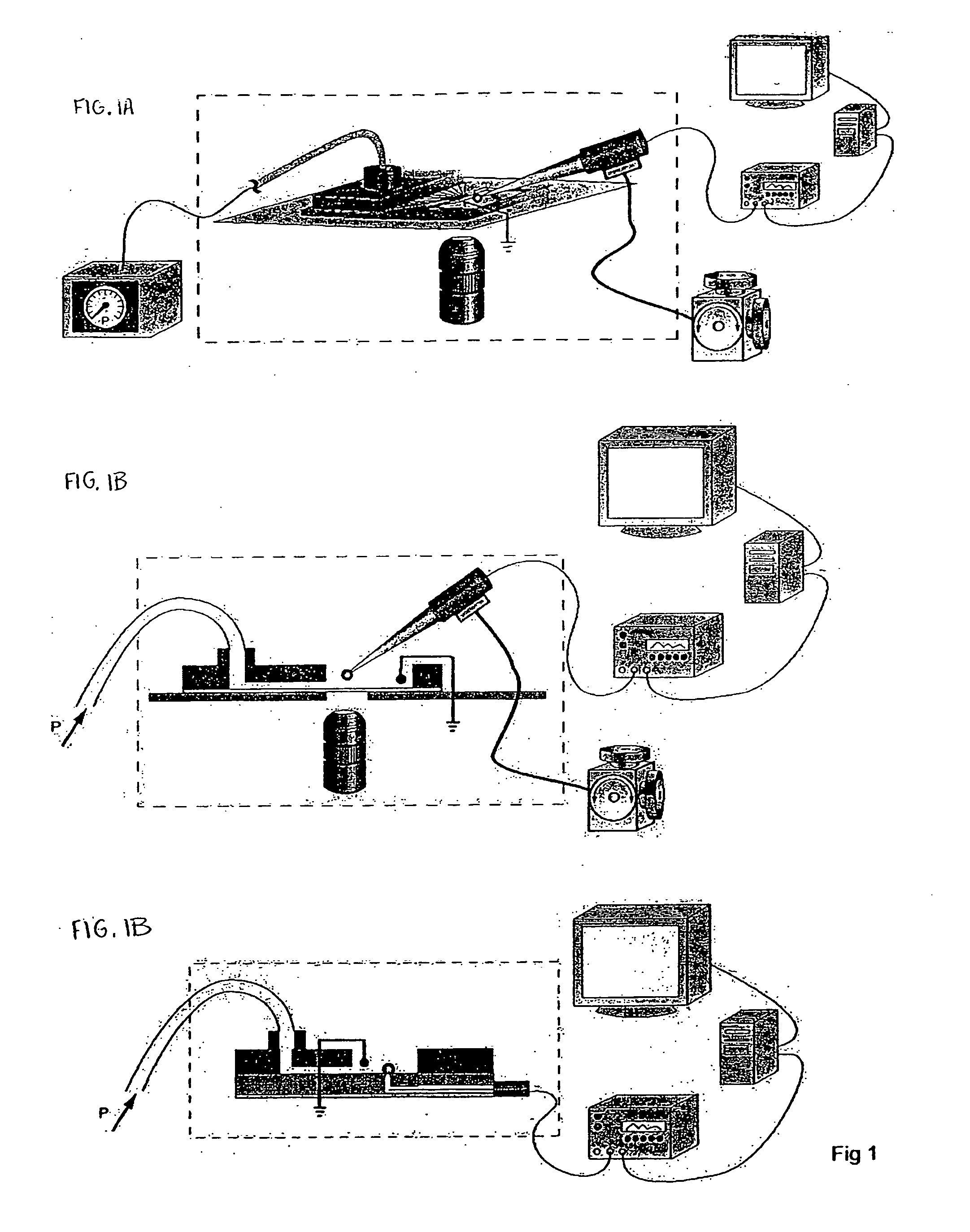
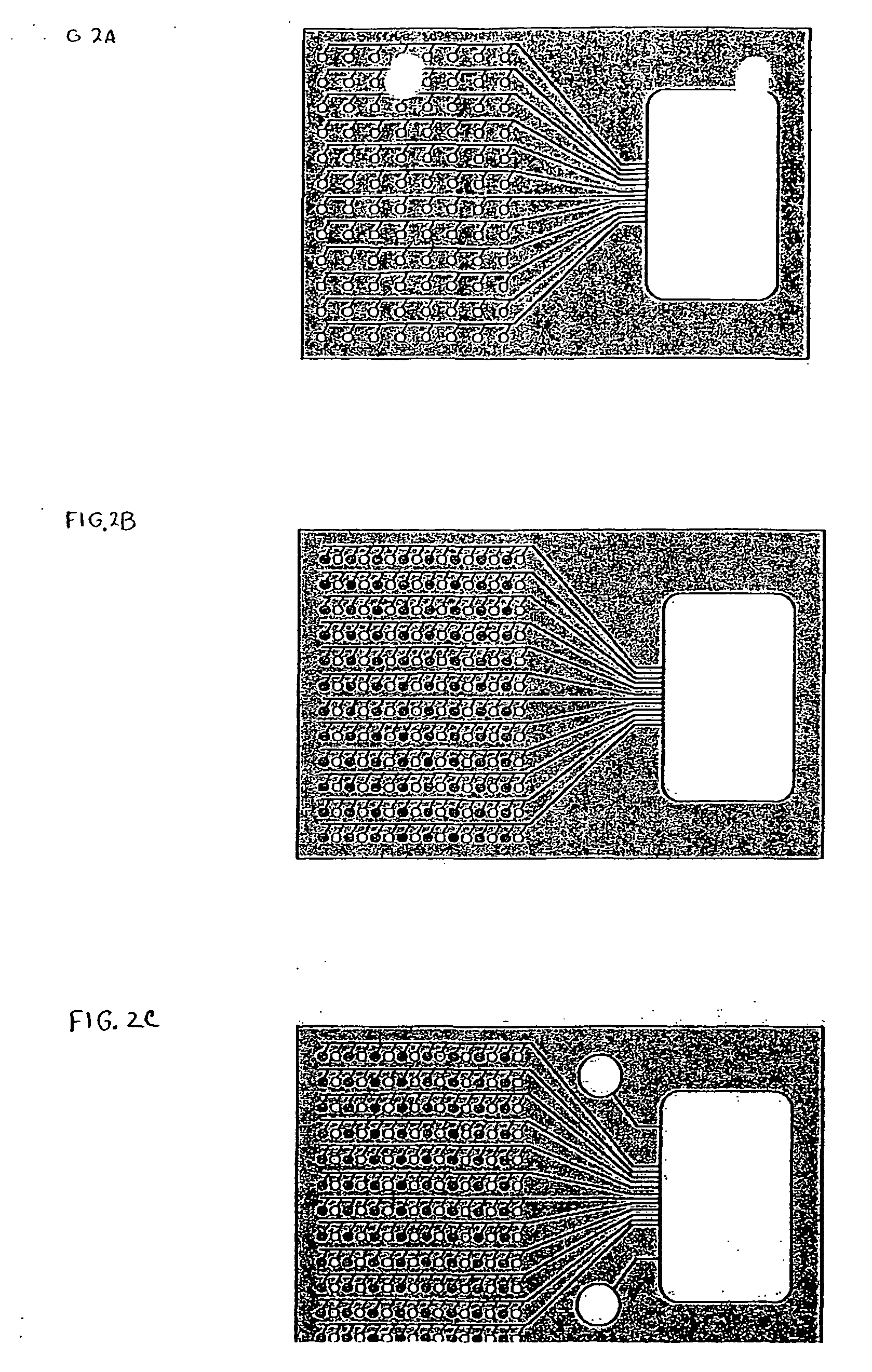
Systems and methods for rapidly changing the solution environment around sensors
a sensor and solution environment technology, applied in the field of systems and methods for rapid and programmable delivery of aqueous streams to sensors, can solve the problems of affecting the development of hts platforms, ignoring significant drug activity of existing hts drug discovery systems targeting ion channels, and avoiding detection of false drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Microfabrication of a Substrate
[0252]FIG. 19 shows examples of microchannels fabricated in silicon by deep reactive ion etching in SF6. Masks for photolithography were produced using standard e-beam writing on a JEOL JBX-5DII electron beam lithography system (medium reflective 4″ chrome masks and Shipley UV5 resists, 50 keV acc. voltage, dose 15 μC / cm−2, exposure current 5 nA). The resist was spin coated at 2000 rpm for 60 s giving 250 nm of resist and soft baked for 10 minutes at 130° C. on a hotplate before exposure. The pattern was post exposure baked for 20 minutes in an oven at 130° C. and developed for 60 s in Shipley MF24-A, rinsed in DI water and etched in a reactive ion etcher (Plasmatherm RIE m-95, 30 s, 50 W, 250 mTorr, 10 ccm O2). The chrome was etched for 1-2 minutes in Balzers chrome etch #4, the mask was stripped of the remaining resist using Shipley 1165 remover and rinsed in acetone, isopropanol and DI water. A 3″, [100], two sides polished, low N-doped Silicon waf...
example 2
Re-Sensitization of Patch-Clamped Cells Using Microfluidic-Based Buffer Superfusion and Cell Scanning
[0256] Microchannels were molded in a polymer, polydimethylsiloxane (PDMS), which were then sealed irreversibly onto a glass coverslip to form an enclosed channel having four walls.
[0257] The procedure used is the following:
[0258] (1) A silicon master used for molding PDMS was fabricated by first cleaning the wafer to ensure good adhesion to the photoresist, followed by spin coating a layer (˜50 am) of negative photoresist (SU 8-50) onto the wafer. This layer of negative photoresist was then soft baked to evaporate the solvents contained in the photoresist. Photolithography with a mask aligner was carried out using a photomask having the appropriate patterns that were prepared using e-beam writing. The exposed wafer was then baked and developed by washing away the unexposed photoresist in an appropriate developer (e.g. propylene glycol methyl ether acetate).
[0259] (2) This develo...
example 3
Rapid Scanning of A Patch-Clamped Cell Across Interdigitated Streams of Ligands and Buffer for HTS Applications
[0269] One preferred embodiment for implementing HTS using the current invention is to scan a patch-clamped cell rapidly across interdigitated streams of buffer and ligands, with each ligand stream corresponding to a different drug. In these applications, as discussed above, both the flow rate of the fluids exiting the microchannels and the scan rate of the patch-clamped cell are important FIGS. 21A-D show the response of patch-clamped whole cells after being scanned across the outlets of a 7-channel structure. The width of each channel is 100 μm, the thickness is 50 μm, and the interchannel spacing is 25 μm. This 7-channel structure is identical to that shown in FIG. 16B. The procedure used for fabricating the microchannels and for patch clamping are identical to that described in Example 2 (see above). The patch clamped cell used was a PC-12 cell, which was placed betwee...
PUM
| Property | Measurement | Unit |
|---|---|---|
| angle | aaaaa | aaaaa |
| angle | aaaaa | aaaaa |
| thick | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com