Modified Viral Particles with Immunogenic Properties and Reduced Lipid Content Useful for Treating and Preventing Infectious Diseases
a technology of immunogenic properties and modified viral particles, which is applied in the direction of immunological disorders, antibody medical ingredients, drug compositions, etc., can solve the problems of severe damage to the body, extreme suffering, morbidity and mortality, and enormous economic burden on society, and achieves simple, effective and efficient immunologic response.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
A. Delipidation of Serum Produces Duck Hepatitis B virus (DHBV) Having Reduced Infectivity
[0130]A standard duck serum pool (Camden) containing 106 ID50 doses of DHBV was used. ID50 is known to one of ordinary skill in the art as the infective dosage (ID) effective to infect 50% of animals treated with the dose. Twenty-one ducklings were obtained from a DHBV negative flock on day of hatch. These ducklings were tested at purchase and shown to be DHBV DNA negative by dot-blot hybridization.
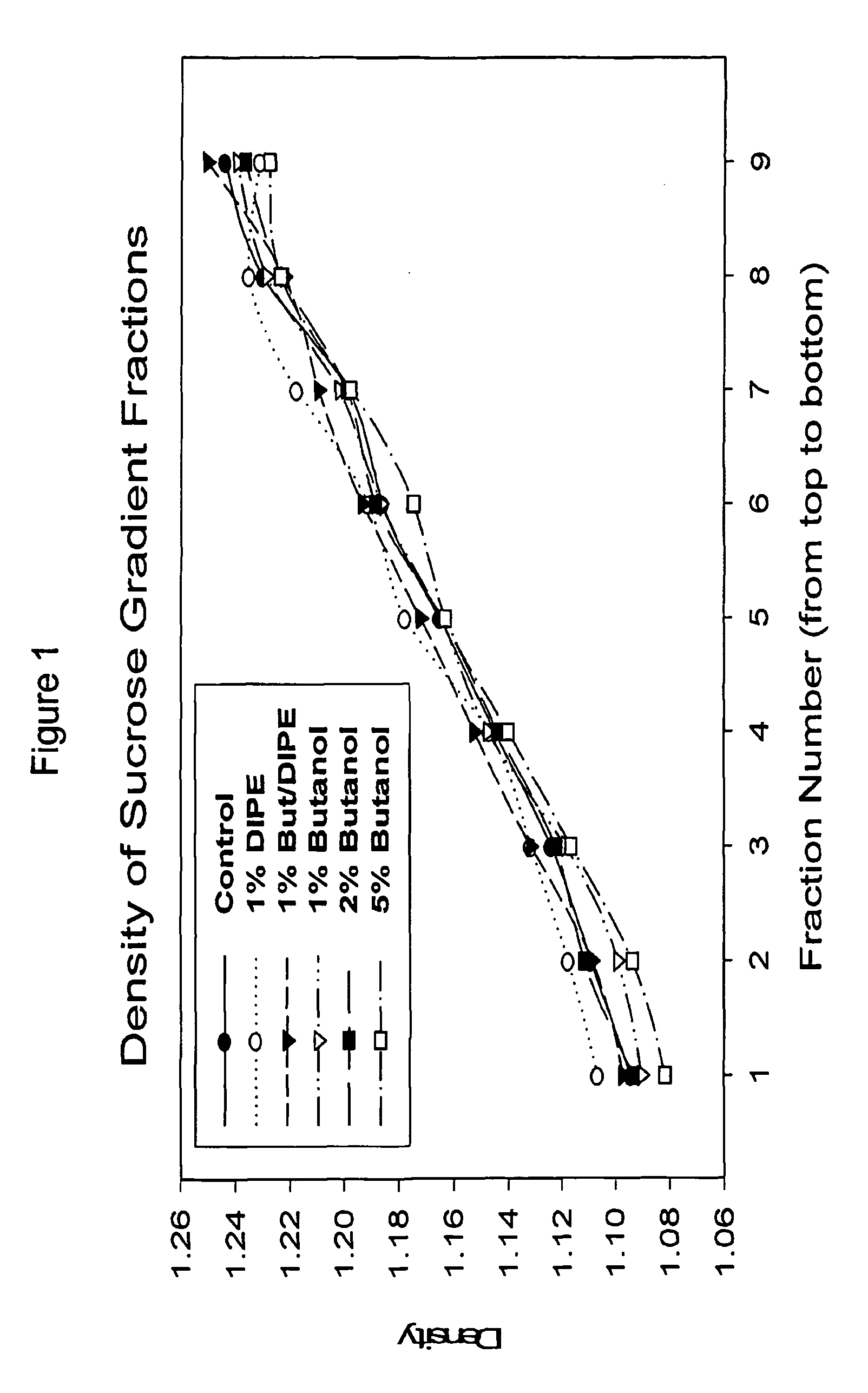
[0131]The organic solvent system was mixed in the ratio of 40 parts butanol to 60 parts diisopropyl ether. The mixed organic solvent system (4 ml) was mixed with the standard serum pool (2 ml) and gently rotated for 1 hour at room temperature. The mixture was centrifuged at 400×g for 10 minutes and the lower aqueous phase (containing the plasma) removed at room temperature. The aqueous phase was then mixed with an equal volume of diethyl ether and centrifuged as before to remove any remaining lipid / s...
example 2
A. Delipidation of Cattle Pestivirus (bovine viral diarrhea virus, BVDV), as a Model for Hepatitis C
[0145]A standard cattle pestivirus isolate (BVDV) was used in these experiments. This isolate, “Numerella” BVD virus, was isolated in 1987 from a diagnostic specimen submitted from a typical case of ‘Mucosal Disease’ on a farm in the Bega district of New South Wales (NSW), Australia. This virus is non-cytopathogenic, and reacts with all 12 of a panel of monoclonal antibodies raised at the Elizabeth Macarthur Agricultural Institute (EMAI), NSW, Australia, as typing reagents. Therefore, this virus represents a ‘standard strain’ of Australian BVD viruses.
[0146]The Numerella virus was grown in bovine MDBK cells tested free of adventitious viral agents, including BVDV. The medium used for viral growth contained 10% adult bovine serum derived from EMAI cattle, all of which tested free of BVDV virus and BVDV antibodies. This serum supplement has been employed for years to exclude the possibi...
example 3
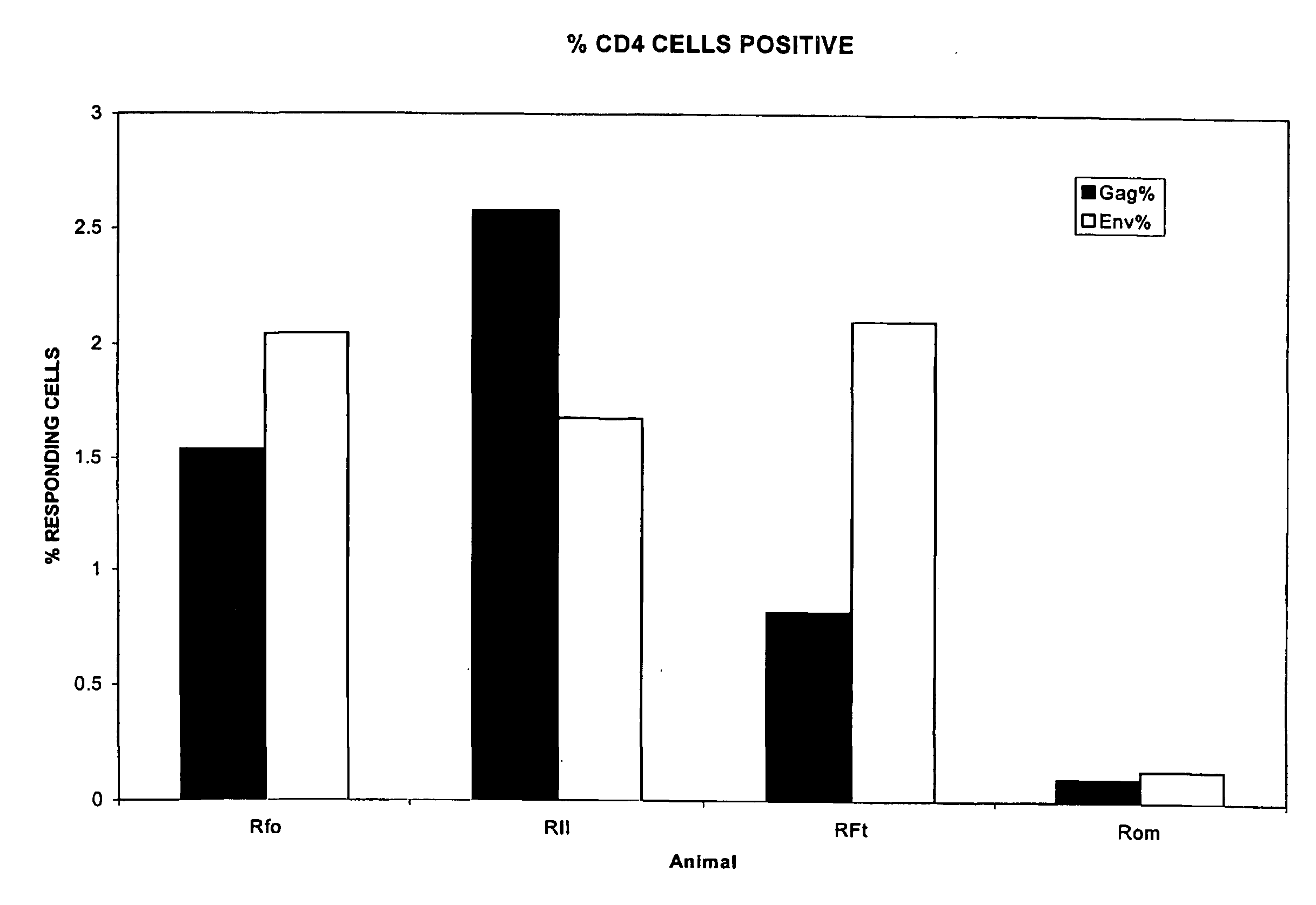
Use of Delipidated SIV to Induce or Augment SIV Specific Humoral and CD4+ T Cell Memory Responses in Mice—a Model for a New Auto-vaccination Strategy Against Lentiviral Infection
[0159]The following studies focused on the simian equivalent of human HIV, termed SIV. The purpose was to utilize delipidated-SIV mac251 (an uncloned highly pathogenic isolate of SIV) to carry out studies to determine the relative immunogenicity of the delipidated virus in mice. The complete nucleotide sequence of an infectious clone of simian immunodeficiency virus of macaques, SIVmac239, has been determined. Virus produced from this molecular clone causes AIDS in rhesus monkeys in a time frame suitable for laboratory investigation. The proviral genome including both long terminal repeats is 10,279 base pairs in length and contains open reading frames for gag, pol, vif, vpr, vpx, tat, rev, and env. The nef gene contains an in-frame premature stop after the 92nd codon. At the nucleotide level, SIVmac239 is c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com