Polypeptide, DNA molecule encoding the polypeptide, vector, preparation method and use
a dna molecule and polypeptide technology, applied in the field of biotechnology, can solve the problems of serious consequences of amputation, influence on appearance as well as physical and psychological health, and complex mechanisms, and achieve the effects of no side effects, promotion of tissue repair and wound healing, and promotion of skin tissue repair
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of the Gene for the Broad-Spectrum Antiseptic Active Polypeptide from Chaerilus tryznai Kovarik
[0152]A: Extraction of Total RNA from the Venom Gland of Chaerilus tryznai Kovarik (Trizol LS One-Step Method: Trizol LS was Purchased from Invitrogen, USA)
[0153](1) 500 mg of the venom gland from the scorpion's tail was ground into fine powders in liquid nitrogen, added with 10 mL of TRIZOL reagent with well mixing, and placed at room temperature (20-25° C., the same as hereinafter) for 5 min. (2) 2 mL chloroform was then added with mixing for 15 s, placed at room temperature for 2-3 min, and centrifuged at 12000 g and 4° C. for 15 min. (3) The aqueous phase was taken out and added with same volume of isopropanol, then placed at room temperature for 10 min, and centrifuged at 12000 g and 4° C. for 10 min to obtain RNA precipitate. (4) The precipitate was washed with 5 ml of 75% ethanol and centrifuged at 7500 g for 5 min. (5) The RNA precipitate was dried followed by dissolut...
example 2
Structure Analysis of Ctryamp Polypeptide and the Structurally Homologous Amphiphilic Polypeptides Thereof
[0170]According to the sequence (FIRIARLLRIF) of the mature peptide of Ctryamp as set forth in SEQ ID NO: 2 as provided in EXAMPLE 1, the secondary structure of Ctryamp was predicted by using on-line NPS@ server [DSC method (Discrimination of protein Secondary structure Class)], and shown by software AHTHEPROT 2000. Results showed that, the Ctryamp contained 100% of α-Helix structure, had a typical amphiphilic α-Helix structure and comprised a large number of basic residues (Arg) with net positive charges. Based on the helix diagram of the polypeptide sequence, a large number of point mutations on the polypeptide sequence FIRIARLLRIF of Ctryamp were performed then. It was found that the sequence FIX1IAX2LLX3IF (X1, X2 and X3 independently are any one of three basic amino acids His, Arg and Lys) (SEQ ID NO: 1) did not influence its amphiphilic property (Table 1). Therefore, the p...
example 3
Polypeptide and the Structurally Homologous Amphiphilic Polypeptides Thereof
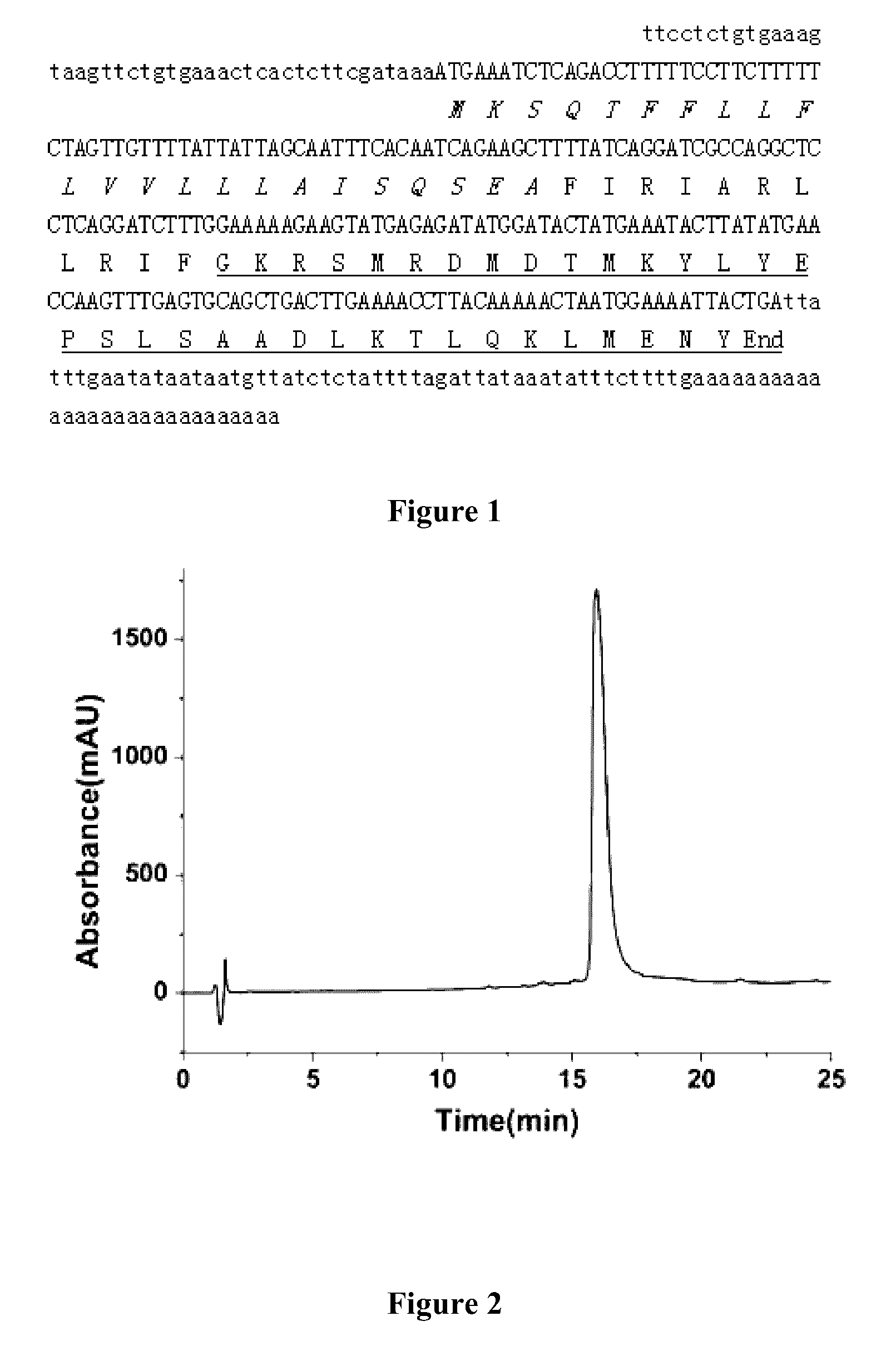
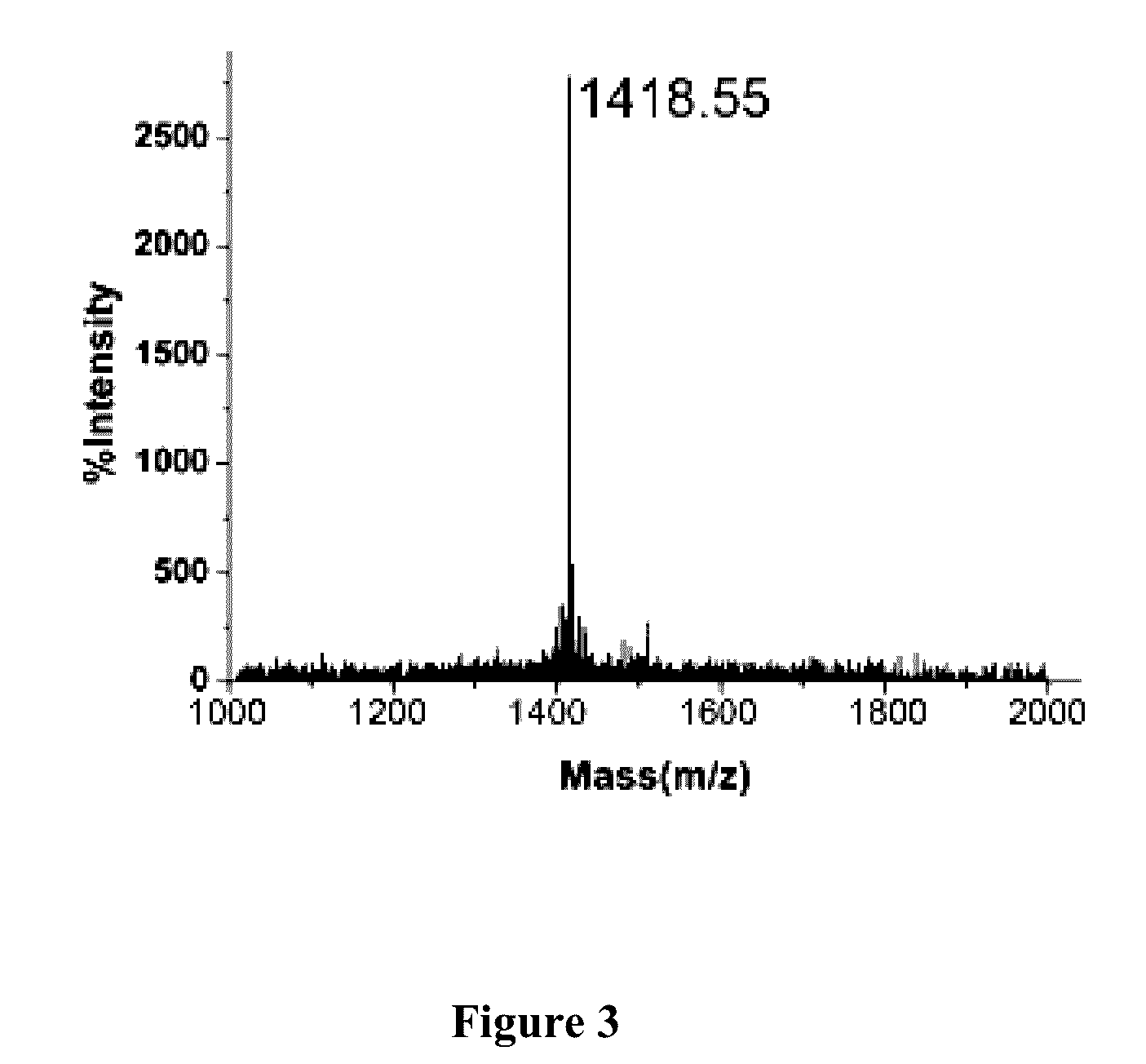
[0171]Artificial synthesis was carried out according to the amino acid sequence of the Ctryamp (FIRIARLLRIF) as provided in EXAMPLE 1 and that of the structurally homologous amphiphilic polypeptides thereof (FIX1IAX2LLX3IF, as provided in EXAMPLE 2). A high-purity Ctryamp polypeptide (as shown in FIGS. 2 and 3) and structurally homologous amphiphilic polypeptides thereof were obtained by solid-phase chemical synthesis (Table 1).
Example 4
Bacteriostasis Experiment of Ctryamp Polypeptide and the Structurally Homologous Amphiphilic Polypeptides Thereof
[0172]Bacteriostasis Experiment on Gram-Negative Bacteria:
[0173]96-well plate culturing method: (1) When Pseudomonas aeruginosa (including a standard strain, clinically isolated strain and drug resistant strain), Escherichia coli (including standard strains CCTCC AB94012 and ATCC25922, a clinically isolated strain and drug resistant strain), Klebsiella pneumoniae, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com