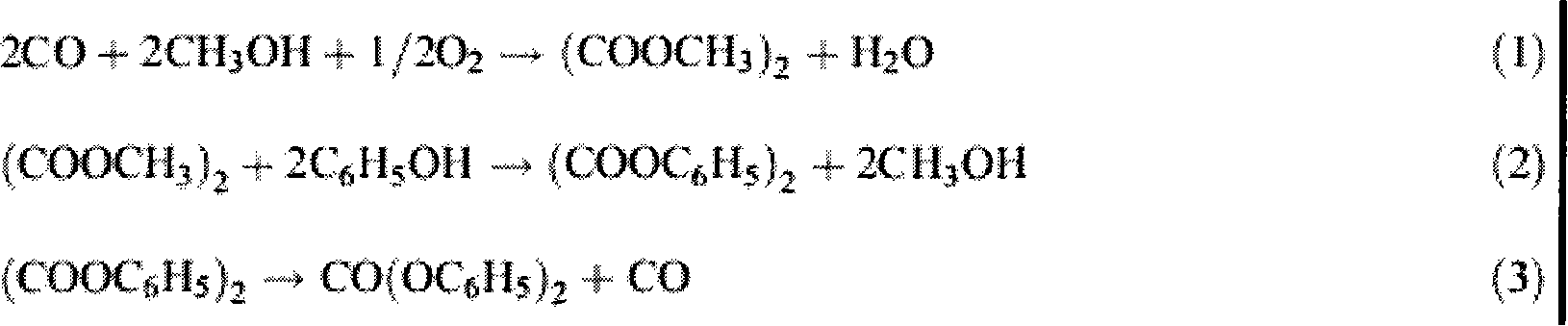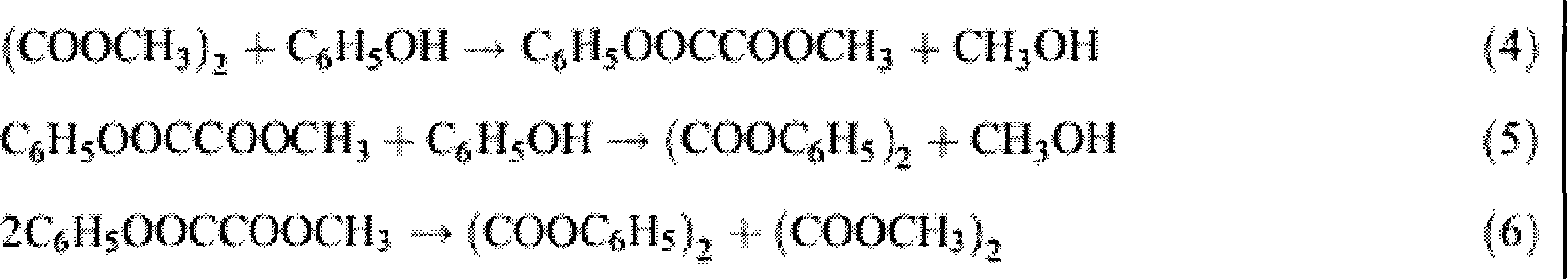Organic acid base catalyst for synthesizing aryl ester carboxylic acid by interesterification
A technology for synthesizing aryl carboxylate and catalyst, which is applied in the fields of organic chemistry and catalytic chemistry, can solve the problems of slow reaction rate, long production and operation cycle, easy loss of active center metal ions, etc., and achieve good catalyst performance and mild operating conditions , the effect of short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The embodiment of the present invention is to investigate under the catalytic reaction conditions of the catalyst of the present invention, using dimethyl oxalate and phenol as an example to prepare methyl phenyl oxalate and diphenyl oxalate. The reaction process is the same as that in the background technology. (4)-(6) The reaction steps are the same.
[0024] The specific process is carried out in a 100ml three-necked flask, heated by heat-collecting electromagnetic stirring, equipped with a thermometer to display the temperature of the reaction system. The consumption of technical grade dimethyl oxalate is 17 millimoles, the consumption of analytical pure phenol is 85 millimoles, 0.3 gram Cp ' (CH 2 ) 3 -MCM-41 is a heterogeneous catalyst (containing 0.38 mmoles of alkene in 0.3 g of catalyst), added under normal pressure. Stir and heat up, the reaction temperature is controlled at 180±2°C, and the reaction time is 2 hours. (4)-(6) The reaction equilibrium constan...
Embodiment 2-5
[0026] With the cyclocene material Cp’(CH) immobilized on Si-MCM-41 2 ) 3-MCM-41 is a heterogeneous catalyst, and the catalyst consumption is 0.3 grams (containing 0.38 millimoles in 0.3 grams of catalysts), and the reaction time is carried out respectively for 4, 6, 8, and 10 hours. Under the circumstances, carry out transesterification reaction and form embodiment 2-5, examine reaction result.
[0027] Table 1: Cp'(CH 2 ) 3 -Reaction evaluation when MCM-41 is used as catalyst
[0028]
Embodiment 6-10
[0030] With 0.38 mmol Cp'(CH 2 ) 3 -MCM-41 is a homogeneous catalyst, the amount of catalyst is 0.38 mmol, and the reaction time is carried out respectively for 2, 4, 6, 8, and 10 hours. Embodiment 6-10, investigate reaction result.
[0031] Table 2: Cp'(CH 2 ) 3 Si(OEt) 3 Reaction evaluation when used as a catalyst
[0032]
[0033] Comparing Examples 1-5 and 6-10, it can be seen that after the immobilization of the cyclocene is not only conducive to the separation of the reaction material and the catalyst, but also under the condition that the activity of the catalyst is kept little changed, the total selectivity of the catalyst to the main product MPO and DPO Improvement, especially in the selectivity of the final product DPO. In Example 5, when reacting for 10 hours, the yield of diphenyl oxalate reached 41.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com