Perylene tetracarboxylic diimide-carbazole-dithienyldiazosulfide conjugated polymer as well as preparation method and application thereof
A perylenetetracarboxylic acid diimide and conjugated polymer technology, which is applied in the field of organic compound synthesis, can solve problems such as low carrier mobility, low conversion efficiency, and ineffective use of carrier electrode collection efficiency. , to achieve the effects of enhancing electron affinity, improving utilization, and being easy to operate and control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
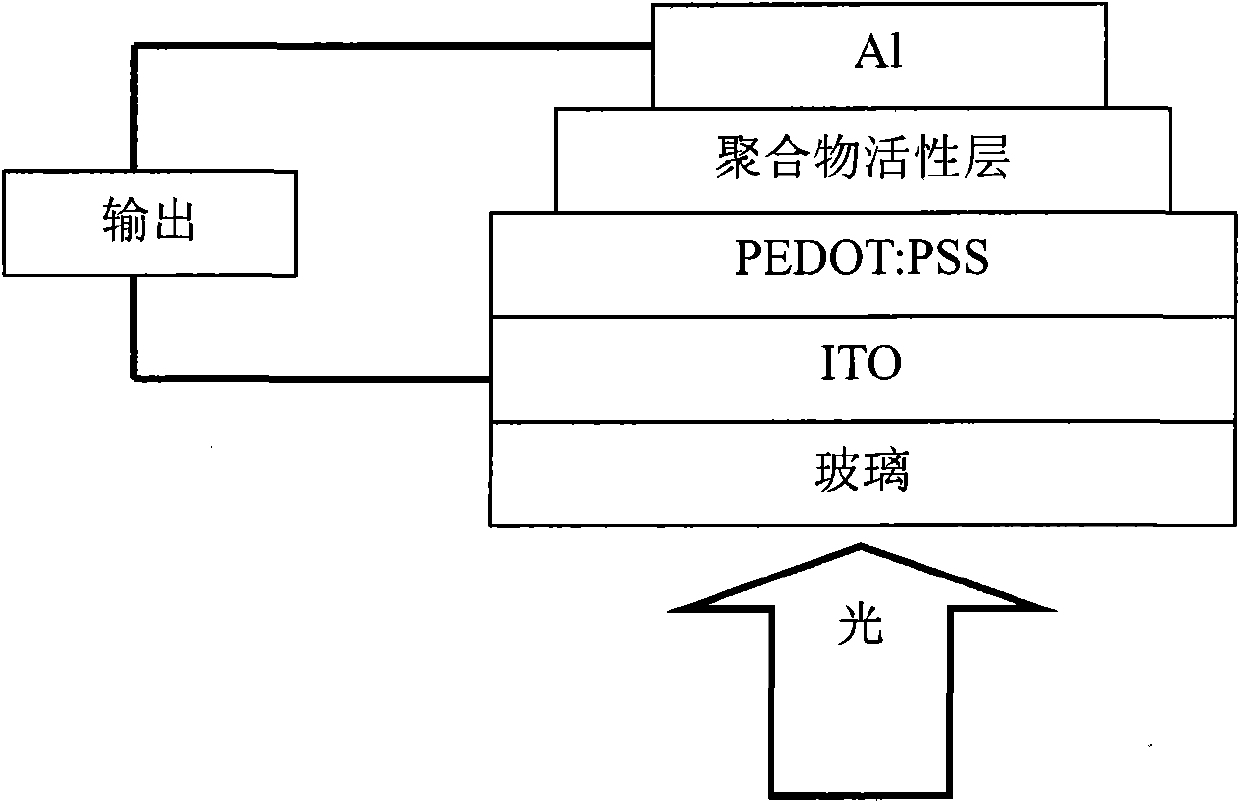
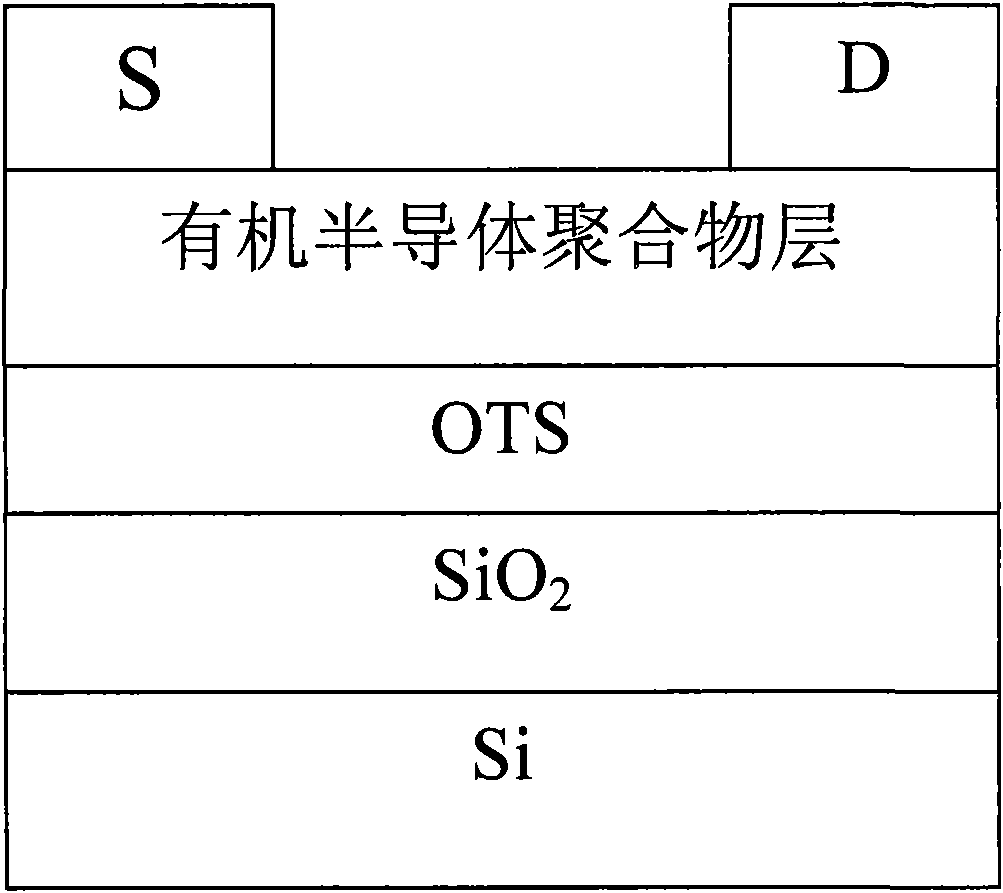
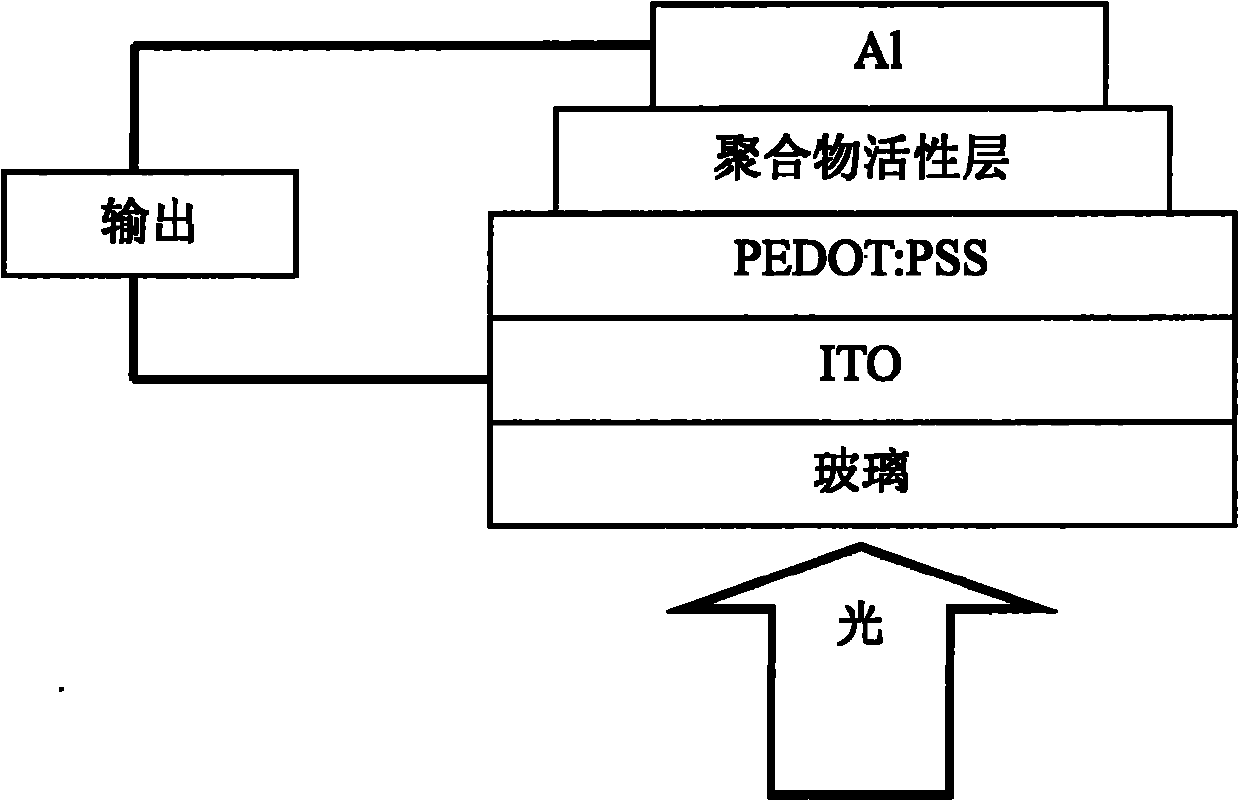
Image
Examples
Embodiment 1
[0039] Preparation of N,N'-two-(3,4,5-tri-dodecyloxybenzene)-1,7-dibromo-3,4,9,10-perylenediimide:
[0040]
[0041] In the reaction flask, add 0.192g 1,7-dibromo-3,4,9,10-perylene tetraanhydride, 0.70g 3,4,5-tri-dodecyloxy-1-aminobenzene and 16ml propane acid, ultrasonically oscillated for 10 minutes to fully mix the reactants, and then pass nitrogen gas into the reactants for 0.5 hours, then stop feeding nitrogen gas, and heat the reactants to 80° C. for 48 hours. After the reaction was completed, cool to room temperature, add chloroform to dissolve, then wash the organic layer with sodium bicarbonate solution to obtain a red suspension, filter, add anhydrous magnesium sulfate to the filtrate to dry to remove water, and finally spin dry. The product was obtained after column separation (dichloromethane:petroleum ether=3:1). MS (EI) m / z: 1806 (M+).
Embodiment 2
[0043] Preparation of 5-tributylstannane-3-hexylthiophene:
[0044]
[0045] Add 5mL 3-hexylthiophene and 50mL THF to a 250mL three-necked flask, then cool down to -30°C with liquid nitrogen / isopropanol, then add 2.5M n-butyllithium dropwise, and react at -30°C for 1 hour, then Join SnBu at one time 3 Cl (96%) 8.5mL, continued to react at -30°C for 0.5 hours, then naturally warmed up to room temperature, and allowed the reaction system to continue to react for 45 hours, then moved the mixture containing the reaction product into ice water, added and extracted with diethyl ether, Finally, dry over anhydrous magnesium sulfate to remove water, remove the solvent under reduced pressure, and use silica gel / petroleum ether (30-60° C.) column chromatography to obtain a colorless and transparent oily liquid.
Embodiment 3
[0047] Preparation of 4,7-bis(3-hexylthiophene)-2,1,3-benzothiadiazole:
[0048]
[0049] Add 1mol of 4,7-dibromo-2,1,3-benzothiadiazole and 2mol of 5-tributylstannane-3-hexylthiophene, 0.03mol of PdCl to a 250mL three-necked flask 2 (PPh 3 ) 2 , 30ml of THF, after reflux reaction under nitrogen protection for 6 hours, then remove the solvent under reduced pressure to obtain a red solid, and then apply the obtained red solid to silica gel / CH 2 Cl 2 / petroleum ether (60-90° C., volume ratio 1:1) column chromatography, and then the column chromatography product was recrystallized with absolute ethanol to obtain orange-red needle-like crystals. MS (EI) m / z: 468 (M+).
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com