Recombined litopenaeus setiferus protein SF-P9, preparation method and application thereof
A SF-P9, shrimp protein technology, applied in the fields of botanical equipment and methods, biochemical equipment and methods, applications, etc., can solve problems such as small molecular weight, and achieve the effect of inhibiting the growth of tumor cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] Example 1 Amplification and cloning of Penaeidin gene in Penaeus vannamei
[0117] 1. Design and synthesis of amplification primers:
[0118] According to the pen-2 gene of shrimp peptide, a pair of primers P1 and P2 were designed. The primers were synthesized by Shanghai Shenggong Biological Engineering Co., Ltd. and purified by PAGE. The nucleotide sequences of P1 and P2 are respectively: upstream primer P1: 5'GAATTCTACAGGGGCGGTTACACA 3'. Downstream primer P2: 5'TCTAGAGCCTTGTCATCGTCATCTCCTTTTACTAAGTGACAACA 3'.
[0119] 2. Extraction of total RNA from Penaeus vannamei and synthesis of the first strand of cDNA:
[0120] The vannamei prawns were raised in a water tank with oxygen at 22°C for later use. Select healthy shrimps in the inter-molt period, rinse them with DEPC-treated sterile water, collect 750μL of hemolymph from the abdominal sinuses of the shrimps with a 2.5mL disposable syringe, add an equal volume of anticoagulant (pH7.0), and inspect under a microscope Count,...
Embodiment 2
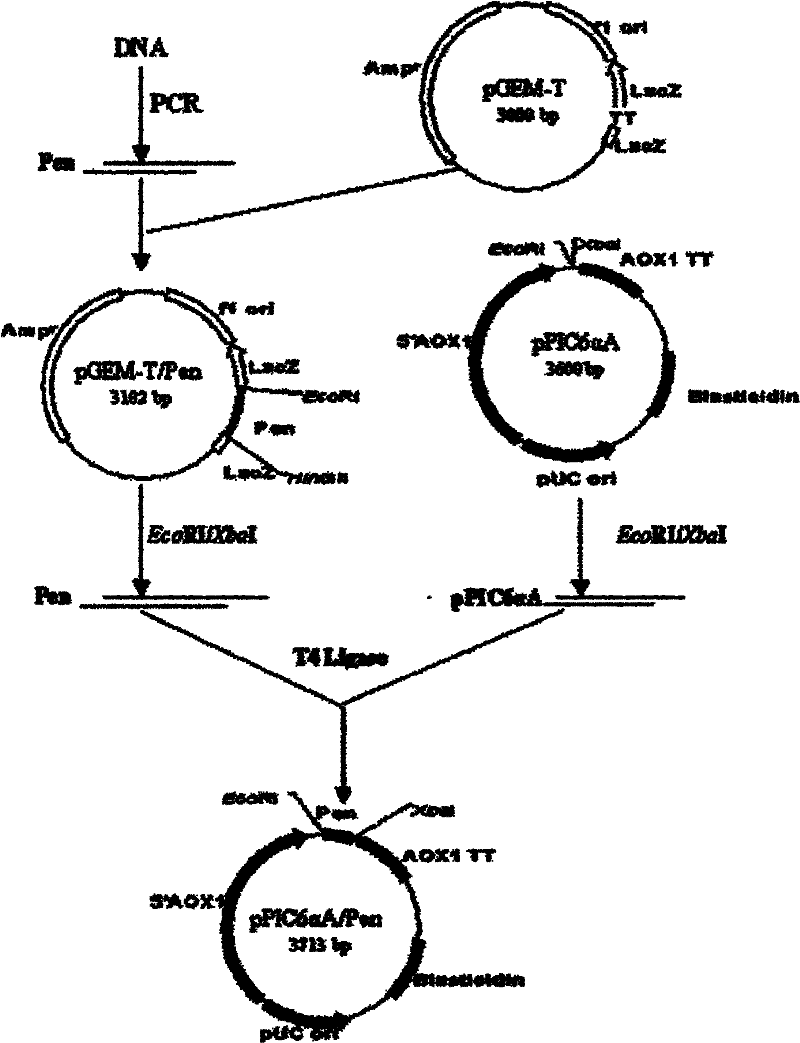
[0151] Example 2 Construction and identification of recombinant shuttle plasmid pPIC6αA / Pen
[0152] 1. Construction of recombinant shuttle plasmid pPIC6αA / Pen:
[0153] Extract the recombinant plasmid pGEM-T / Pen, use Eco RI and Xba I double enzyme digestion to obtain the target fragment Pen; the same digestion pPIC6αA empty vector. After double digestion, the Pen and the empty vector pPIC6αA DNA fragment were exposed to T4DNA ligase at 16°C overnight to obtain the recombinant plasmid pPIC6αA / Pen.
[0154] The connection reaction system is as follows:
[0155] pPIC6αA vector DNA fragment: 2μl; penaeidin DNA fragment: 10μl; T4 DNA buffer: 1.5μl; T4 DNA ligase: 1.5μl.
[0156] Transform Escherichia coli with the recombinant plasmid pPIC6αA / Pen E.coli JM109 competent cells were screened for positive clones on LB plates containing 300μg / ml blasticidin to obtain E. coli strains E.coli JM109 (pPIC6αA / Pen). Use the lysis method to extract plasmid pPIC6αA / Pen DNA (see Molecular Cloning ...
Embodiment 3
[0167] Example 3 Transformation of recombinant shuttle plasmid pPIC6αA / Pen Pichia pastoris X-33
[0168] 1. Linearization of recombinant shuttle plasmid pPIC6αA / Pen DNA:
[0169] Prepare a small amount of recombinant plasmid pPIC6αA / Pen DNA 15-20μg, digest it with RNaseA at 37°C for 30 min, then use restriction enzymes in a 60μL system Sac I Carry out restriction enzyme digestion linearization. After keeping it in a 37°C water bath for 4 hours, extract once with phenol:chloroform (25:24) and once with chloroform:isoamyl alcohol (24:1), add 0.1 volume of 3M acetic acid Sodium (pH5.2), 2.5 times the volume of absolute ethanol, mix well and place it at -20°C for precipitation overnight, centrifuge at 4°C, 12000rpm for 20 minutes, discard the supernatant, and wash twice with 75% ethanol prepared in ultrapure water. After air-drying naturally, dissolve the precipitate with 5-10μL TE solution and store at -20°C for later use. Restriction endonuclease Sac I digested the linearized ve...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com