Preparation method for ferric oxide coated tin dioxide nanometer polycrystalline microballoon
A technology of ferric oxide and tin dioxide, which is applied in the direction of electrical components, battery electrodes, circuits, etc., can solve the problems of low yield, complicated conditions, and high cost, and achieve high yield, uniform size and shape, and synthetic The effect of simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Preparation of tin dioxide and polysaccharide spheres
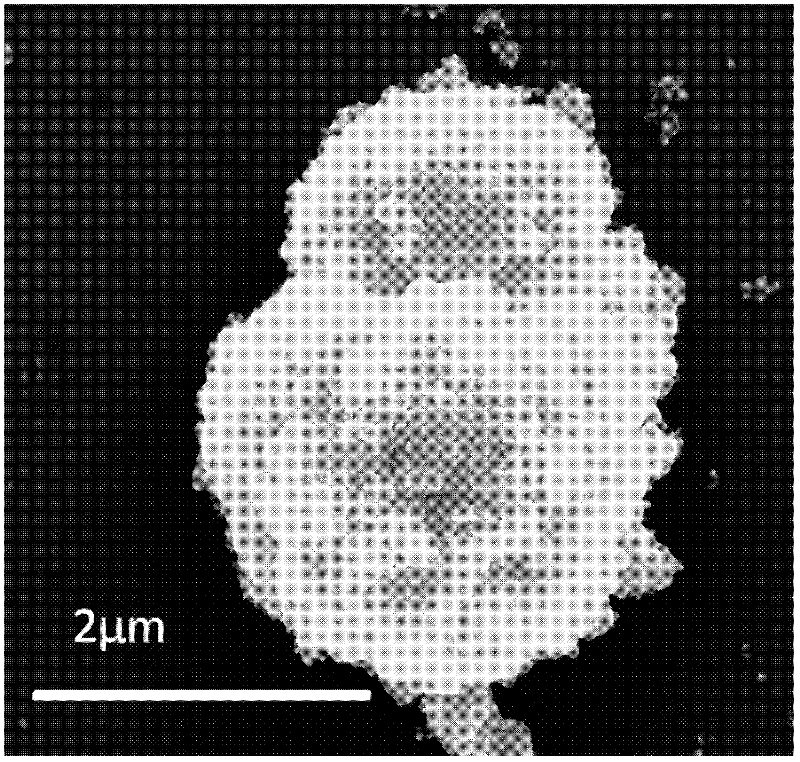
[0021] First weigh 7.3g of SnCl 4 ·5H 2 Dissolve O in 20mL of distilled water, then weigh 7.15g of sucrose as a template and dissolve in 15mL of distilled water, mix the two solutions evenly and put them into a 45ml hydrothermal kettle, then put the reaction kettle into a blast drying oven heated to 180°C 24h in the box for hydrothermal reaction. After the reaction was completed, the hydrothermal kettle was taken out, and after it was cooled to room temperature, the sample was washed by ultrasonic centrifugation to obtain a brown powdery solid, which was sintered in air at 600°C to obtain tin dioxide nano-polycrystalline microspheres, which Scanning electron microscope photos as figure 2 .
Embodiment 2
[0023] Coating ferric oxide on the basis of tin dioxide and polysaccharide spheres
[0024] First weigh 0.72g of FeCl 3 6H 2 O was dissolved in 30mL of ethanol and stirred for 30 minutes, then 0.81g of urea was weighed and dissolved in 6ml of deionized water and stirred for 30 minutes. After the stirring was completed, 150g of tin dioxide, polysaccharide spheres and FeCl 3 ·6H 2 O solution and urea solution were mixed together, stirred for 15 minutes, and then the mixed liquid was subjected to ultrasonic vibration for 15 minutes. Finally, the mixed liquid was put into a 45ml hydrothermal kettle, and heated to 60° C. in a blast drying oven for 48 hours of hydrothermal reaction.
Embodiment 3
[0026] Sintered
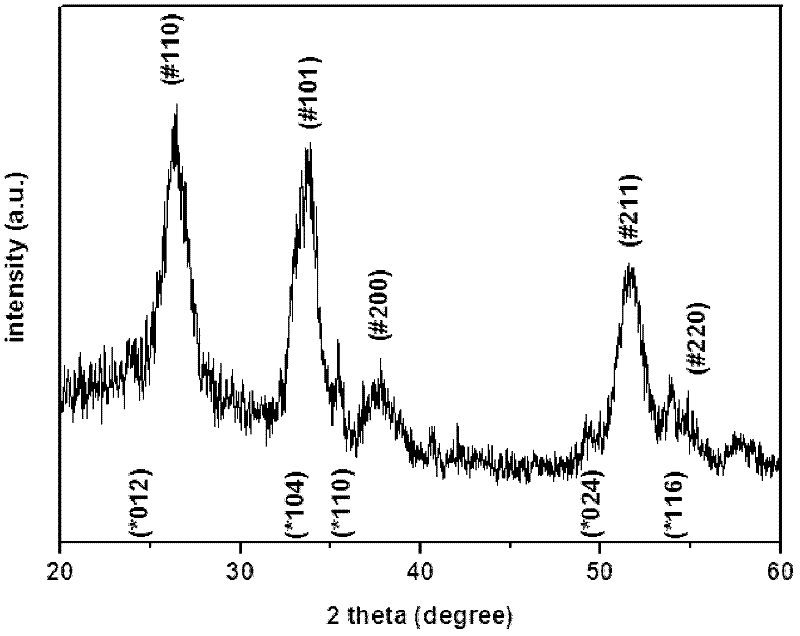
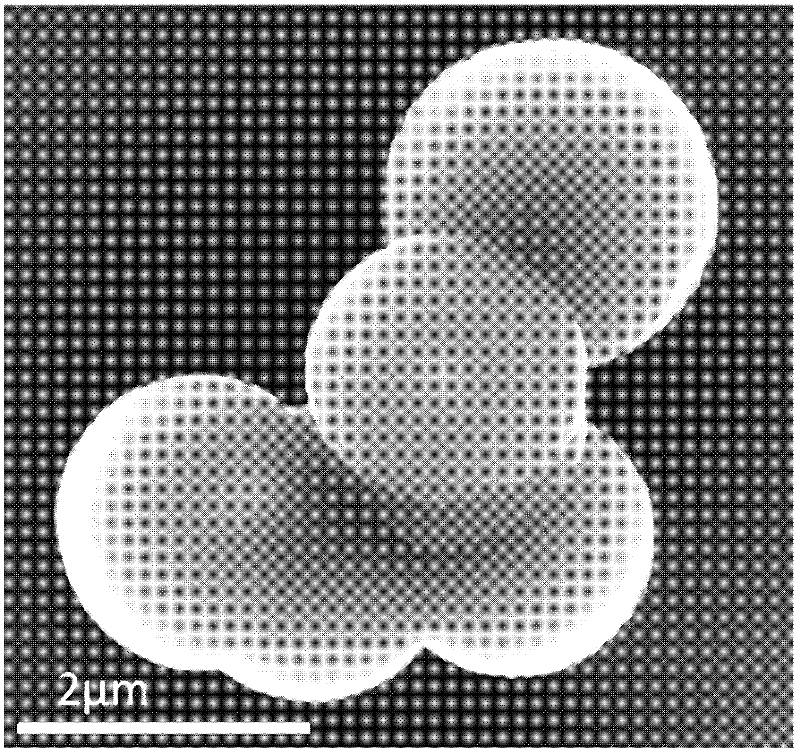
[0027] After the hydrothermal reaction is completed, put the brown powdery solid into the muffle furnace and sinter in the air at 500°C for 4 hours. The carbon and the oxygen in the air react to generate carbon dioxide gas, and finally the red ferric oxide coated carbon dioxide is obtained. Tin nano polycrystalline microsphere solid powder. The X-ray diffraction spectrogram of gained ferric oxide coating tin dioxide nano-polycrystalline microsphere is as follows figure 1 , and its scanning electron micrograph is shown in image 3 . Gained solid powder was ultrasonicated for 30 minutes, and its scanning electron micrograph was as follows: Figure 4 , it can be seen that the ferric oxide on the surface of some microspheres falls off, exposing the tin dioxide microspheres inside.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com