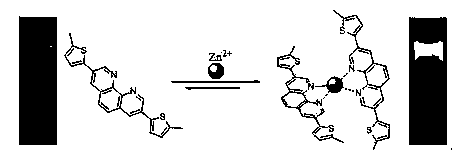
3,8-bis-(5-methyl-2-thienyl)-1,10-phenanthroline and its preparation method and application in zinc ion fluorescence sensing and cell imaging
A phenanthroline and thiophene-based technology, applied in the 3,8-position of 1,10-phenanthroline to expand the field of bis-5-methylthiophene compounds, can solve time-consuming and labor-intensive, difficult to achieve real-time detection of living body detection, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1. Synthesis of 3,8-bis-(5-methyl-2-thienyl)-1,10-phenanthroline
[0022]Add 0.71 g (29.21 mmol) of activated magnesium chips, 5 mL of anhydrous tetrahydrofuran and a catalytic amount of iodine into a 100 mL dry three-necked flask, and keep stirring at room temperature under the protection of argon. Dissolve 1.7 mL (15.10 mmol) of 2-bromo-5-methylthiophene in 5 mL of anhydrous tetrahydrofuran to make a solution, then slowly drop about 10% solution into the above-mentioned three-necked bottle, and continue to drop in after the reaction is triggered remaining solution, and keep the reaction system at a slight boil. After dripping, heat to reflux for 30 min. The reaction solution was cooled to room temperature, poured into a 50 mL dropping funnel, and then slowly dropped into Ni(dppp)Cl 2 and 50 mL of dry tetrahydrofuran in a three-necked flask, stirred at room temperature for 2 h after dropping, then heated to reflux for 12 h, cooled to room temperature, and...
Embodiment 2
[0028] Example 2. Imaging of HL-60 cells
[0029] HL-60 (human acute leukemia cells) suspension was used in DMEM medium, plus 10% FBS (fetal calf serum), penicillin (100 μg·mL -1 ), streptomycin (100 μg·mL -1 ), at 37°C and 5% CO 2 Cultivated under atmosphere. In the experiment, the suspension was centrifuged and washed with Hank's balanced salt solution (HBSS), then resuspended with HBSS, and incubated with 50 μM PHT1 at 37 °C for 45 min. After incubation, the remaining PHT1 was washed away with HBSS, and divided into two culture flasks, with 1 mL of cell suspension in each flask. One of the bottles added Zn 2+ Solution ([Zn 2+ ] = 50 μM), and then the two bottles of suspension were incubated at 37 °C for 20 min. Cell imaging of the two vials of suspension was performed separately using a laser confocal scanning microscope (TCS SP5, Leica, Germany): a 405 nm laser diode was used for the excitation light source, and a 450 nm–500 nm filter was used for emission light co...
Embodiment 3
[0031] Example 3. Imaging of HepG-2 cells
[0032] HepG-2 (human liver cancer cell) suspension was used in DMEM medium, plus 10% FBS (fetal calf serum), penicillin (100 μg·mL -1 ), streptomycin (100 μg·mL -1 ), at 37°C and 5% CO 2 Cultivated under atmosphere. Before staining, wash twice with DMEM medium, resuspend with 10 μM PHT1 solution, and incubate at room temperature for 30 min. After incubation, the remaining PHT1 was washed away with DMEM, and divided into two culture flasks, with 1 mL of cell suspension in each flask. One of the bottles added Zn 2+ Solution ([Zn 2+ ] = 10 μM) and incubated at room temperature for 30 min. Finally, HepG-2 cells were treated with zinc ion chelator (TPEN). Cell imaging was performed using a laser confocal scanning microscope: a 405 nm laser diode was used for the excitation light source, and a 450 nm-500 nm filter was used for emission light collection.
[0033] The experimental results and conclusions of HepG-2 cell imaging are ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com