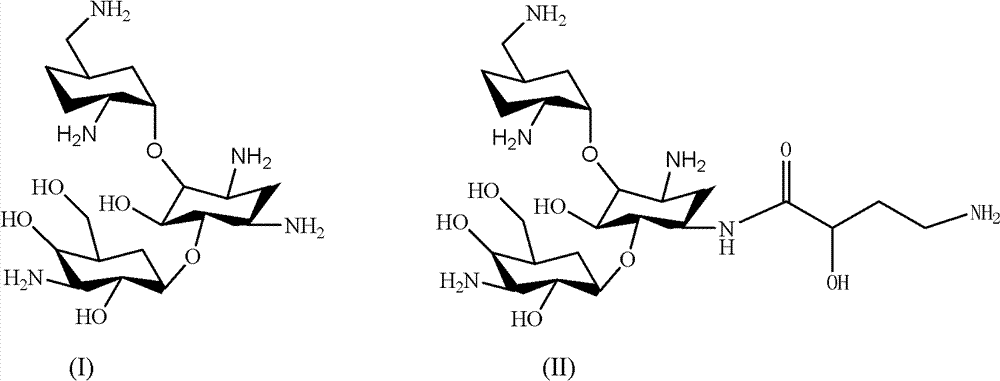
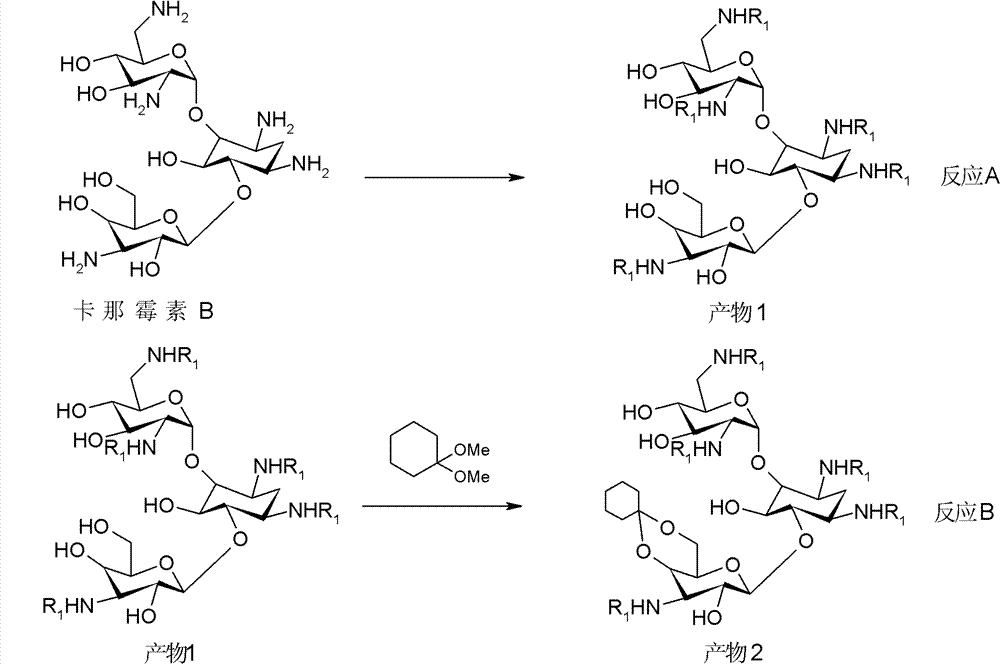
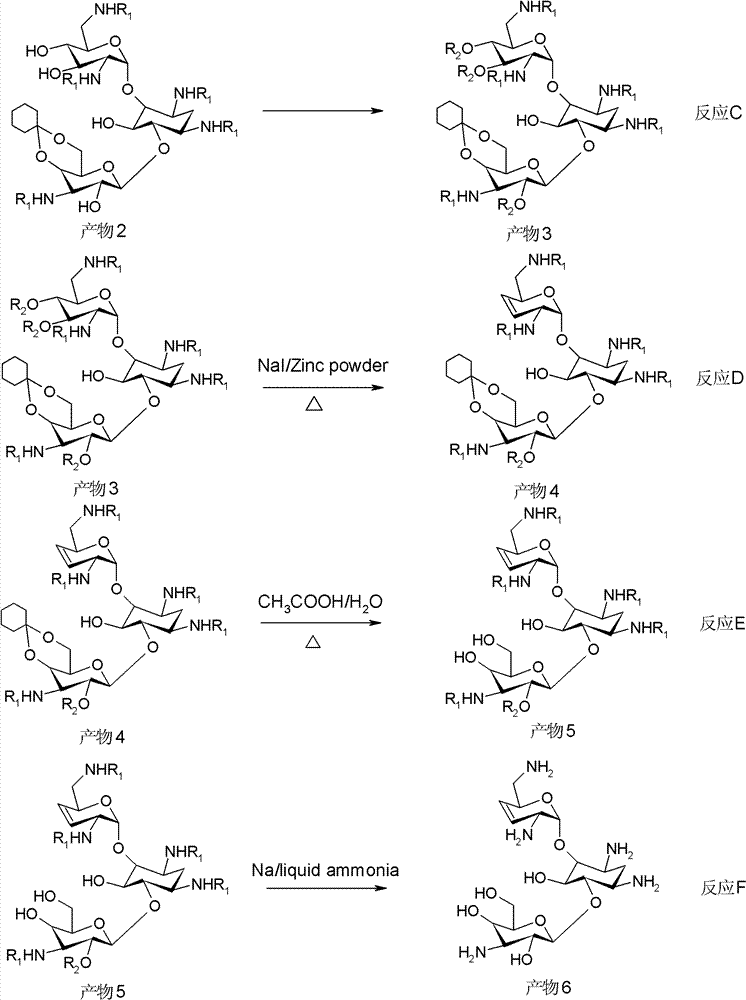
New synthetic method of arbekacin and intermediate of dibekacin thereof
A technology for debekacin and a synthesis method, which is applied in chemical instruments and methods, organic chemistry, sugar derivatives, etc., can solve the problems of affecting yield, low yield of product 7, environmental pollution, etc., and achieves improved reaction activity, The effect of reducing environmental pollution and concise synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] (1) Weigh 10g of kanamycin B and 11g of anhydrous sodium carbonate, dissolve them in 50ml of water, then add 50ml of isopropanol, then weigh 27g of di-tert-butyl dicarbonate and add it, and the system is at 40°C , reacted for 10 hours, filtered the system, and collected the filtrate to obtain 18.3 g of penta-nitrogen-tert-butoxycarbonyl-kanamycin B, with a yield of 90%.
[0100](2) Weigh 18.3 g of penta-nitrogen-tert-butoxycarbonyl-kanamycin B, 1.7 g of anhydrous p-toluenesulfonic acid, dissolve it in 100 ml of N, N-dimethylformamide, add 8.4 ml1, 1-dimethoxycyclohexane, react the system at 50°C for 12 hours, evacuate the system with a vacuum pump for 30 minutes, stop the reaction, pour the system into 1L water to disperse, filter, collect the filtrate, and obtain penta-nitrogen -tert-butoxycarbonyl-4", 6"-oxy-cyclohexylidene-kanamycin B 19.8 g, yield 90%.
[0101] (3) Weigh 19.8g of penta-nitrogen-tert-butoxycarbonyl-4", 6"-oxygen-cyclohexylidene-kanamycin B, dissolve...
Embodiment 2
[0113] In step (1), the molar ratio of di-tert-butyldicarbonate, sodium carbonate and kanamycin B is 6: 8: 1, and other steps are the same as in Example 1 to obtain five-nitrogen-tert-butoxycarbonyl-carboxylate Namycin B 16.3g, yield 80%.
Embodiment 3
[0115] In step (1), the molar ratio of di-tert-butyldicarbonate, sodium carbonate and kanamycin B is 5: 8: 1, and other steps are the same as in Example 1 to obtain five-nitrogen-tert-butoxycarbonyl-carboxylate Namycin B 14.6g, yield 72%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com