Reaction type phosphorus-nitrogen fire retardant and preparation method thereof
A phosphorus-nitrogen flame retardant and reactive technology, applied in the field of reactive phosphorus-nitrogen flame retardants and their preparation, can solve the problem of poor compatibility between small molecule flame retardants and polymers, affecting the mechanical properties of substrates and bonding methods Complicated issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
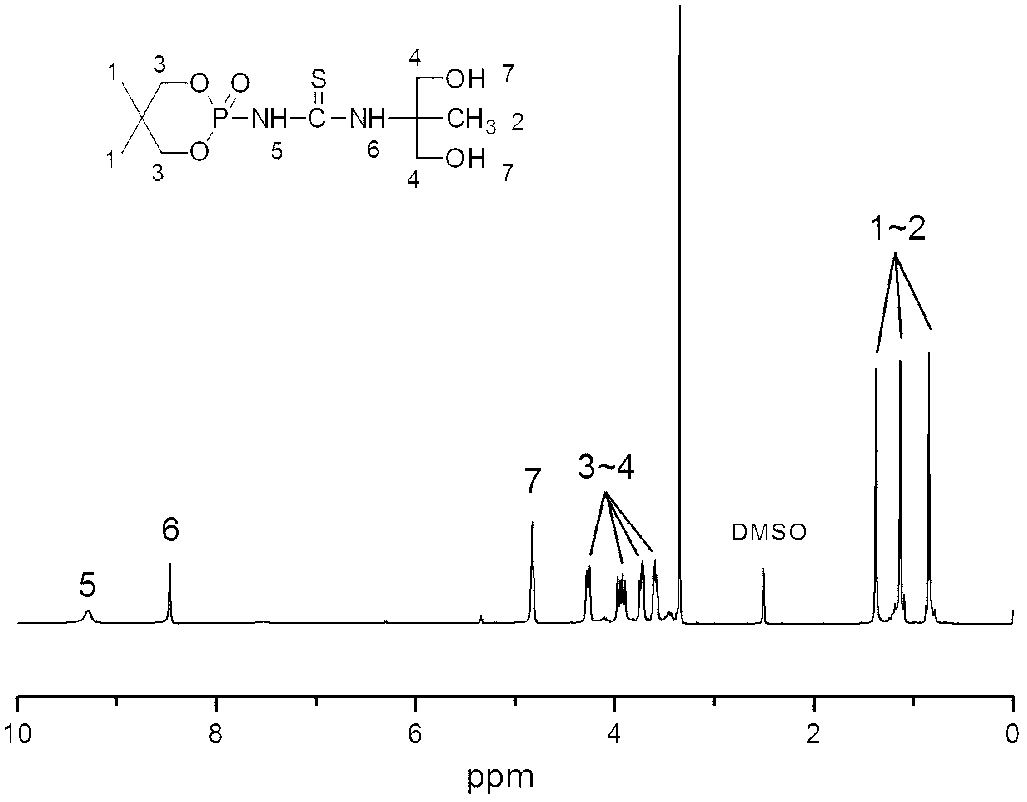
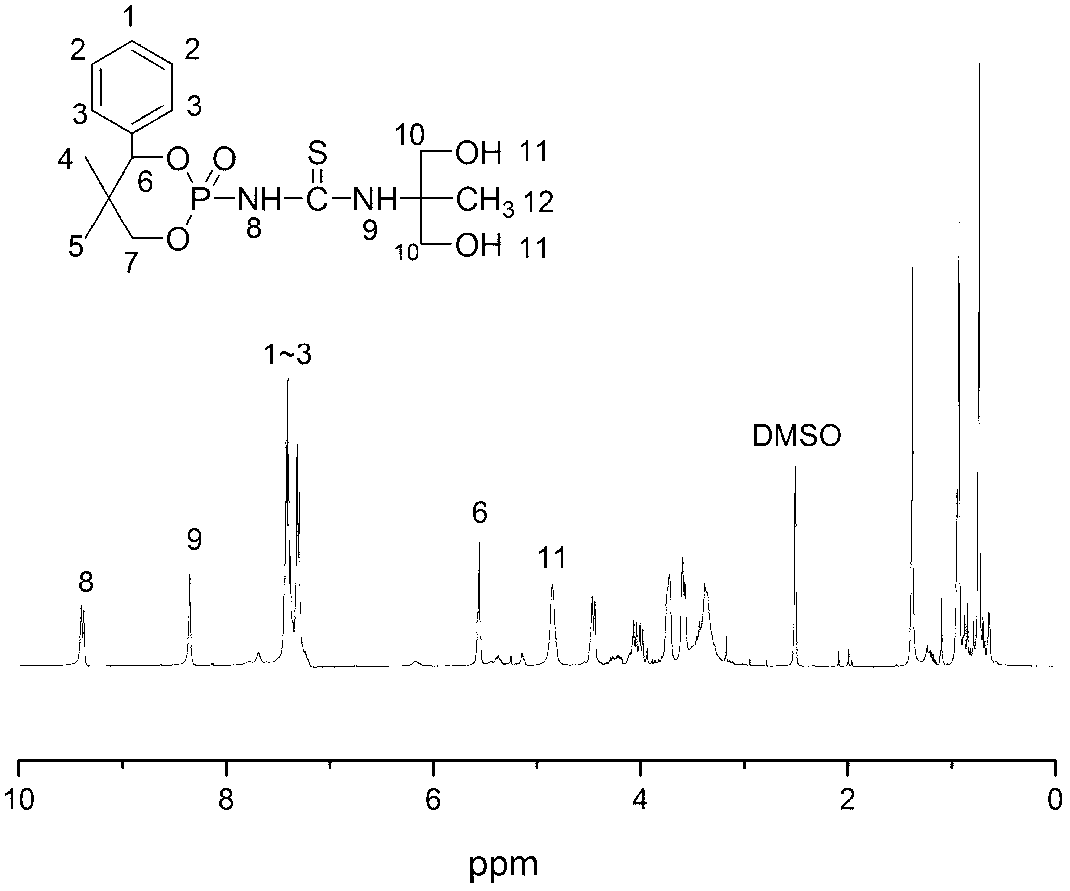
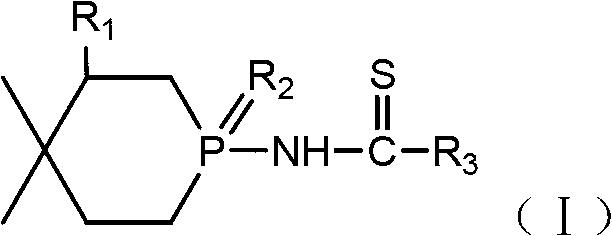
Image
Examples
Embodiment 1
[0076] First, 47.4g (0.44mol) of benzaldehyde and 64.0g (0.88mol) of isobutyraldehyde were stirred and mixed, and 24.8g (0.44mol) of potassium hydroxide was dissolved in 400ml of ethanol and slowly added dropwise to the system through a dropping funnel. The reaction is exothermic. When the temperature reaches 70℃, the dripping is stopped. When the temperature of the system is quickly cooled to 50℃ with a water bath, the ethanol solution of potassium hydroxide is added rapidly. After the dripping is completed, the system is heated and kept at 50-60℃ React for 5 hours. After the reaction was completed, the yellow viscous crude product was obtained by rotary evaporation, which was extracted with chloroform / water system. The obtained chloroform layer was dried with anhydrous magnesium sulfate. After drying, the clear liquid was rotary evaporated to remove chloroform, and recrystallized with toluene / n-hexane system to obtain 61.0 g (0.34mol) 1-phenyl-2,2-dimethyl-1,3-propanediol.
[...
Embodiment 2
[0079] The operation steps for the synthesis of 1-phenyl-2,2-dimethyl-1,3-propanediol used in this example are the same as in Example 1. Add 36.0g (0.20mol) of 1-phenyl-2,2-dimethyl-1,3-propanediol to 200ml of xylene to dissolve it, and then slowly add 19.9g (0.13) dropwise through a constant pressure funnel while stirring at room temperature. mol) Phosphorus oxychloride, the hydrogen chloride gas generated by the reaction is absorbed by sodium bicarbonate aqueous solution, and after the dripping is completed, the temperature is raised to 75°C to react for 2h. After the reaction is completed, the xylene is removed by rotary evaporation, and the obtained solids are respectively n-hexane and anhydrous ether After successive washing, 23.4 g (0.09 mol) of 4-phenyl-5,5-dimethyl-1,3,2-dioxacaprolactone phosphoryl chloride was obtained. Dissolve 23.4g (0.09mol) of 4-phenyl-5,5-dimethyl-1,3,2-dioxacaprolone phosphoryl chloride in 150ml of acetonitrile, and then take 9.7g (0.10mol) of t...
Embodiment 3
[0081] The operation steps for the synthesis of 1-phenyl-2,2-dimethyl-1,3-propanediol used in this example are the same as in Example 1. Add 36.0g (0.20mol) of 1-phenyl-2,2-dimethyl-1,3-propanediol to 200ml of acetone to dissolve it, and then add 21.4g (0.14mol) slowly through a constant pressure funnel while stirring at room temperature ) Phosphorus oxychloride, the hydrogen chloride gas generated by the reaction is absorbed with sodium bicarbonate aqueous solution, after the dripping is completed, the temperature is raised to 56°C for 3 hours, and the acetone is removed by rotary evaporation after the reaction. Then 28.6 g (0.11 mol) of 4-phenyl-5,5-dimethyl-1,3,2-dioxacaprolactone phosphoryl chloride was obtained. Dissolve 28.6g (0.11mol) of 4-phenyl-5,5-dimethyl-1,3,2-dioxacaprolone phosphoryl chloride in 150ml of acetone, and then take 11.6g (0.12mol) of thiocyanate Potassium is dissolved in 50ml of acetone, then the temperature is raised to 53℃, and the dissolved potassi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com