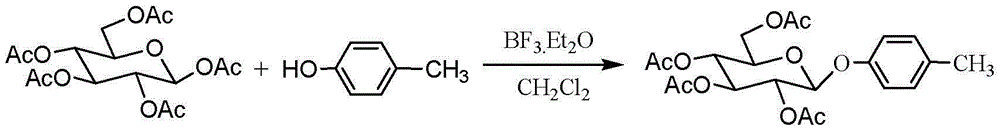Method for chemically synthesising gastrodin
A technology for chemical synthesis and gastrodin, applied in chemical instruments and methods, organic chemistry, bulk chemical production, etc., can solve the problems of high cost, pollution and low total yield, avoid hazards and pollution, and simplify purification operations , the effect of short reaction period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
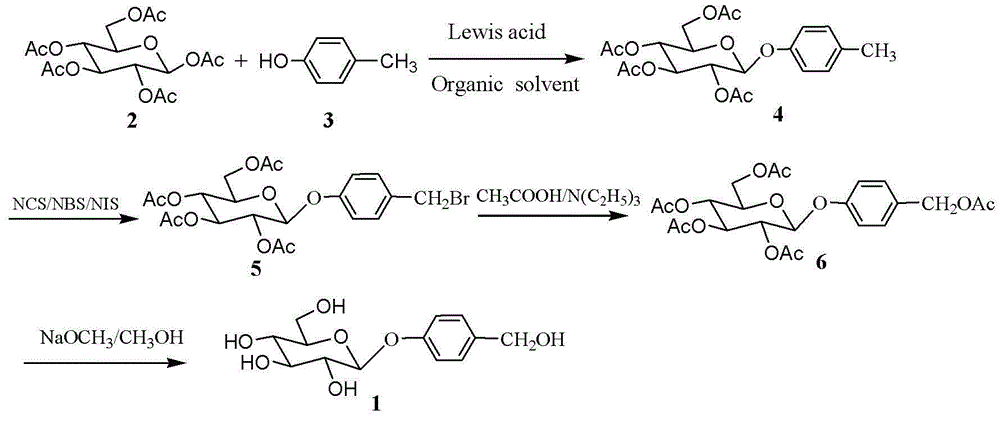
Examples
Embodiment 1
[0029]
[0030]Pentaacetyl-β-D-glucose (50 mmol, 19.5 g ) and p-cresol (60 mmol, 12.9 g ) were dissolved in 150 mL of dichloromethane, 30 g of 4 A molecular sieves were added, and stirred for 1 hour under nitrogen protection. Then boron trifluoride ether solution (30 mmol, 3.58ml) was added dropwise, stirred at room temperature for 4 hours, molecular sieves were removed by filtration with diatomaceous earth, the filter cake was washed with 50mL of dichloromethane, and the filtrate was washed with 300mL of water and 300mL of saturated sodium bicarbonate in sequence solution, washed with 300mL water, the dichloromethane layer was separated, dried with anhydrous sodium sulfate, filtered, the filtrate was concentrated under reduced pressure to obtain a light yellow solid, recrystallized from absolute ethanol to obtain white crystal 4-methylphenyl-2,3,4 , 6-O-tetraacetyl-β-D-glucopyranoside 11.4g, yield 52%, melting point 117-118°C.
[0031] 1 HNMR (500MHz, CDCl 3 ) δ 7.09 ( ...
Embodiment 2
[0042] Pentaacetyl-β-D-glucose (50mmol, 19.5g ) and p-cresol (60 mmol, 12.9g ) were dissolved in 150mL of dichloromethane, 30g of 4 A molecular sieves were added, stirred for 1 hour under nitrogen protection, and then Add tin tetrachloride solution (30 mmol, 3.5 ml) dropwise, stir at room temperature for 4 hours, filter with diatomaceous earth to remove molecular sieves, wash the filter cake with 50 mL of dichloromethane, and dissolve the filtrate with 300 mL of water, 300 mL of saturated sodium bicarbonate, Wash with 300mL of water, separate the dichloromethane layer, dry it with anhydrous sodium sulfate, filter, concentrate the filtrate under reduced pressure to obtain a light yellow solid, recrystallize from absolute ethanol to obtain a white crystal 4-methylphenyl-2,3,4,6 -O-tetraacetyl-β-D-glucopyranoside 9.42g, yield 43%.
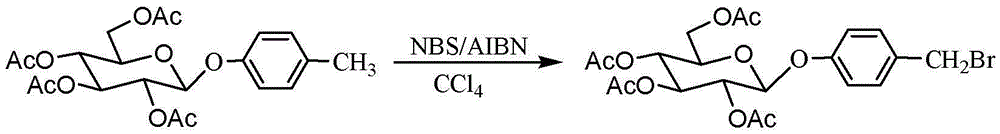
[0043] Dissolve the above 4-methylphenyl-2,3,4,6-O-tetraacetyl-β-D-glucopyranoside (20mmol, 8.73g) in 60mL carbon tetrachloride, add N-bromo Subsuc...
Embodiment 3
[0047] Pentaacetyl-β-D-glucose (25 mmol, 9.75g ) and p-cresol (37.5 mmol, 8.05g ) were dissolved in 75 mL of dichloromethane, 15 g of 4 A molecular sieves were added, and stirred for 1 hour under nitrogen protection. Then boron trifluoride ether solution (30 mmol, 3.58ml) was added dropwise, stirred at room temperature for 4 hours, the molecular sieve was removed by diatom filter, the filter cake was washed with 30mL of dichloromethane, and the filtrate was washed with 150mL of water and 150mL of saturated sodium bicarbonate solution , washed with 150 mL of water, the dichloromethane layer was separated and dried with anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure to obtain a light yellow solid, which was recrystallized from absolute ethanol to obtain a white crystal 4-methylphenyl-2,3,4, 6-O-tetraacetyl-β-D-glucopyranoside 6.89g, yield 63%.
[0048] Dissolve the above 4-methylphenyl-2,3,4,6-O-tetraacetyl-β-D-glucopyranoside (10mmo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com