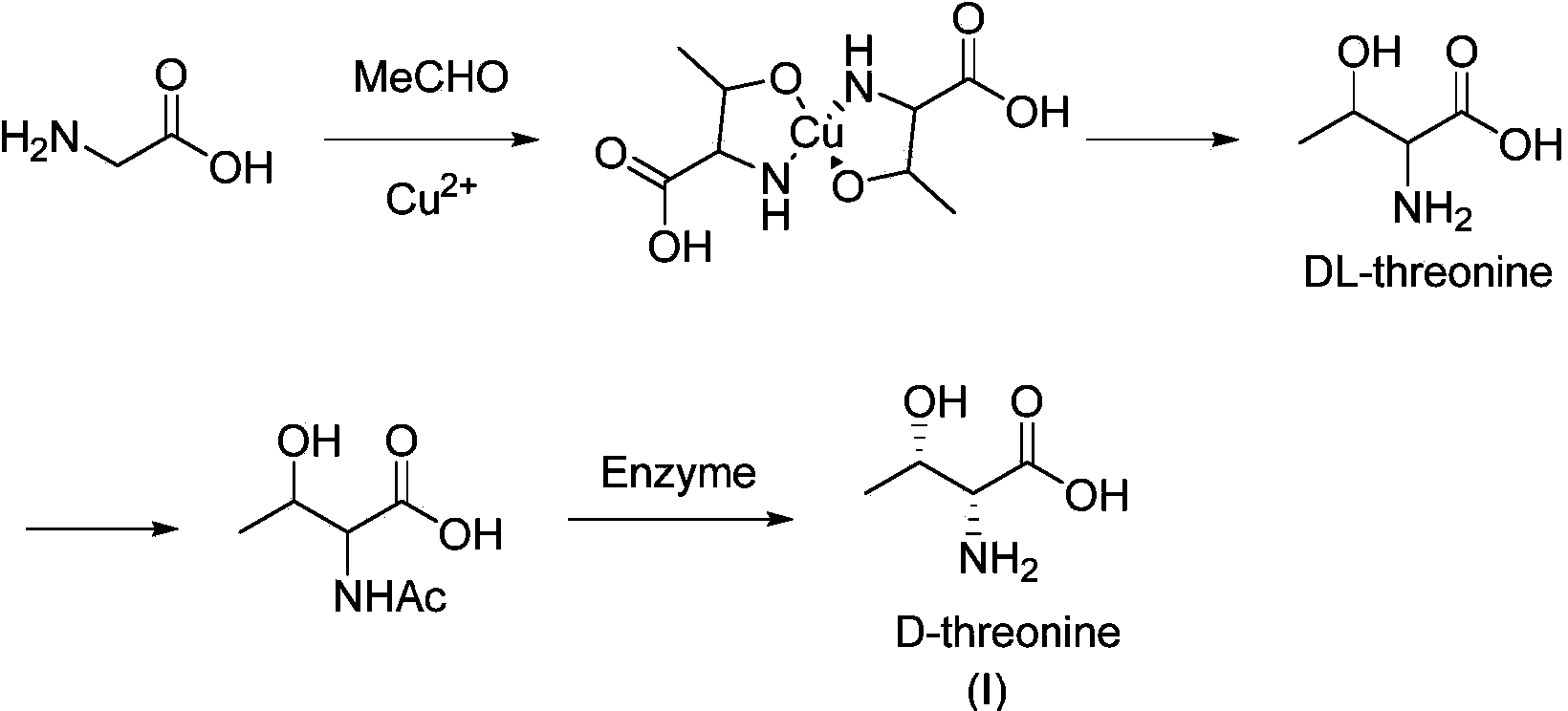
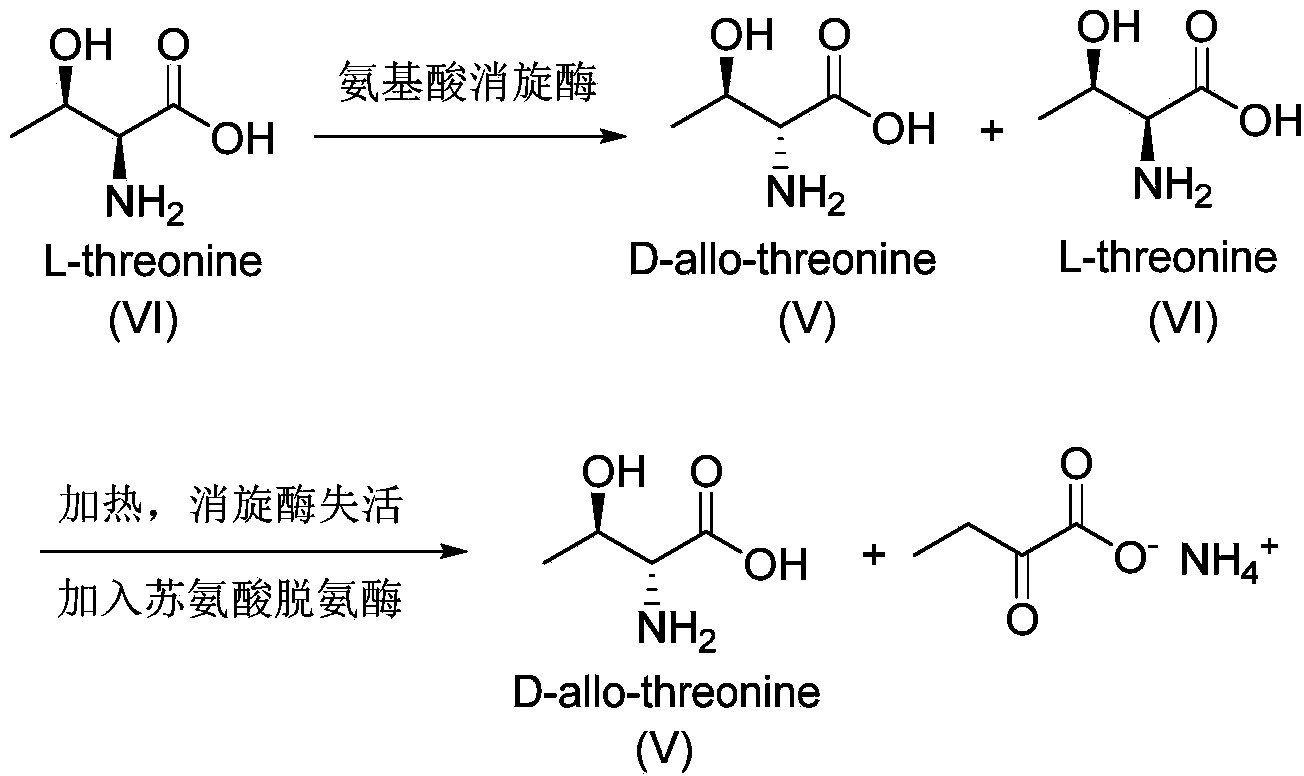
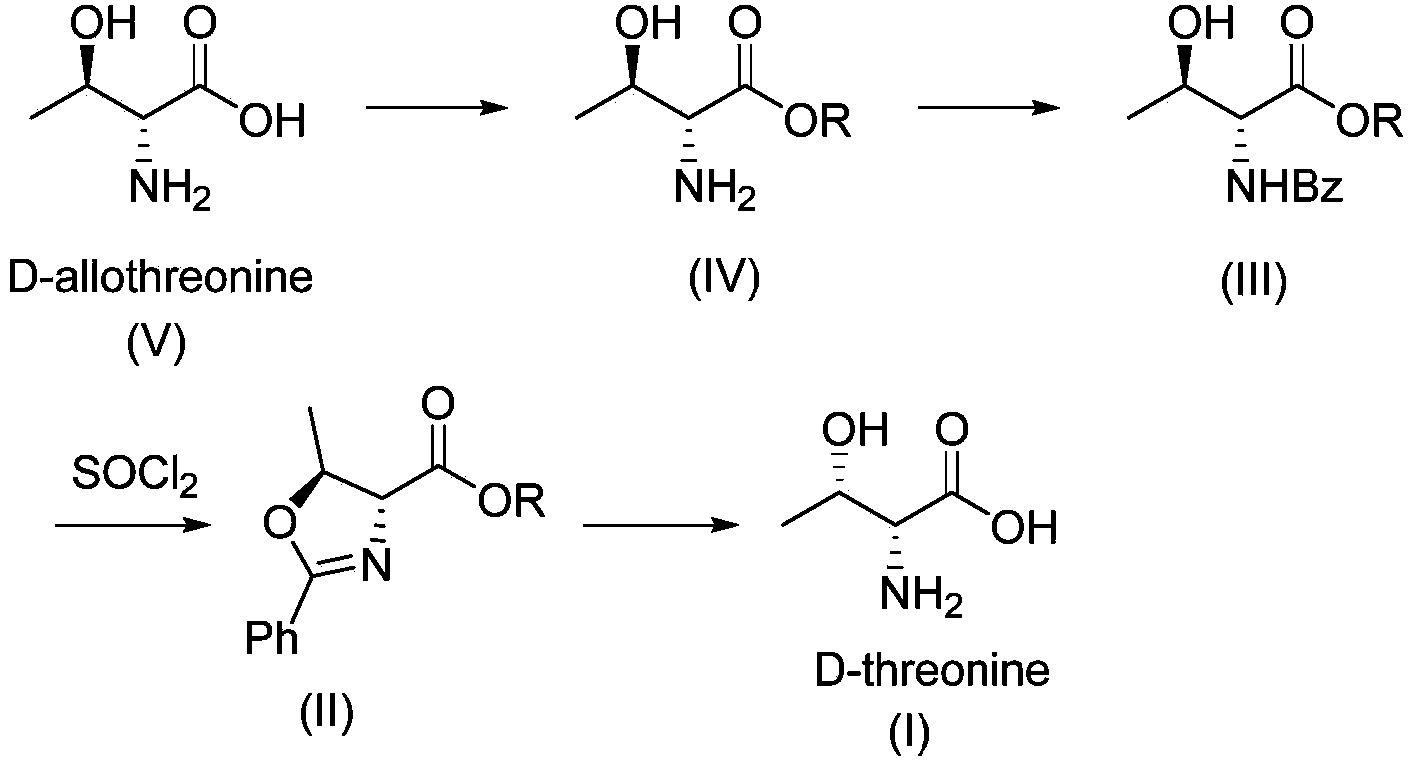
Synthesis method of D-threonine
A synthesis method and threonine technology are applied in the field of synthesis of non-natural amino acid D-threonine, and can solve the problems of only 50% utilization rate of intermediates, low yield of DL-threonine, inconvenient operation and the like, Achieve the effect of being suitable for large-scale popularization and application, low fermentation cost and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1: Construction of racemization of amino acid racemase and expression of L-threonine deaminase
[0056] Main reagents: restriction endonuclease and Taq DNA polymerase (Fermentas), T4 DNA ligase and alkaline phosphatase CIAP (TAKARA), KOD neo plus DNA polymerase (TOYOBO), plasmid extraction kit and mini gel recovery Kit (Axygen), Kanamycin (Shanghai Sangon Bioengineering Company). Primer synthesis, total gene synthesis and subcloning were all entrusted to Nanjing GenScript Biotechnology Co., Ltd.
[0057] Construction of amino acid racemase expression strain:
[0058] According to the reported gene sequence of the amino acid racemase alr derived from Pseudomonas putida KT2440 (NCBI accession number: NC_002947), the sequence was synthesized in the whole gene, and restriction sites NdeI and BamHI were designed at both ends, and subcloned into the vector pET24a to obtain recombinant Plasmid pET24a-alr. The constructed recombinant plasmid pET24a-alr was transforme...
Embodiment 2
[0061] Embodiment 2: the preparation of D-allothreonine
[0062] Shake flask fermentation of two enzyme expression strains: preparation medium TB (12g / L peptone, 24g / L yeast extract, 5g / L glycerol, 2.13g / L KH 2 PO 4 , 16.43g / L K 2 HPO 4 ·3H 2 o). Medium TB was divided into 500mL Erlenmeyer shake flasks with a liquid volume of 100mL, and then heated and sterilized in an autoclave at 121°C for 20min. After cooling, add kanamycin at a final concentration of 100 μg / mL to the sterilized TB medium (prepared as a high-concentration mother solution and filter it to sterilize), and then inoculate the two enzyme-expressing strains into the medium respectively, Cultivate on a shaker at 37°C and 220rpm until OD600=5-6, add 0.2mM IPTG to induce, lower the temperature to 28°C, continue to cultivate for about 24h, centrifuge the fermentation broth at 8000rpm, remove the supernatant, and collect the bacteria for later use.
[0063] Preparation of two enzyme crude enzyme liquids: the cel...
Embodiment 3
[0067] 1. Preparation of D-allothreonine methyl ester (3-2)
[0068]
[0069] Dissolve 6.0 g of D-allothreonine (3-1) in 36 mL of anhydrous methanol, cool the solution to 0°C, add 11.8 g of thionyl chloride dropwise, and react at 0°C for 48 hours, monitor the reaction by TLC After completion, concentrated to obtain 8.5 g of white solid D-allothreonine methyl ester hydrochloride (3-2), with a yield of 99.5%. 1 H NMR (400MHz,MeOD)δ4.26(d,J=6.6Hz,1H),4.09(d,J=3.5Hz,1H),3.85(s,3H),1.27(d,J=6.6Hz,3H ); MS(ESI)m / z=134(M + +1).
[0070] 2. Preparation of N-benzoyl-D-allothreonine methyl ester (3-3)
[0071]
[0072] Under nitrogen protection, dissolve 7.0 g of D-allothreonine methyl ester hydrochloride (3-2) in 35 mL of anhydrous methanol, then add 11.5 g of triethylamine, cool the system to 0°C, and slowly add benzyl Acyl chloride 5.8g, keep warm for 2h after the dropwise addition, TLC monitors that the reaction is complete, evaporate the methanol under reduced pressure, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com