Preparation method of cation exchange resin with high-temperature thermostability
A cation exchange and stability technology, which is applied in the field of cation exchange resin preparation, can solve the problems of ion exchange resin exchange capacity decline, demanding polymerization equipment, low polymerization efficiency, etc., to achieve improved dispersion stability, increased initiation efficiency, high Yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
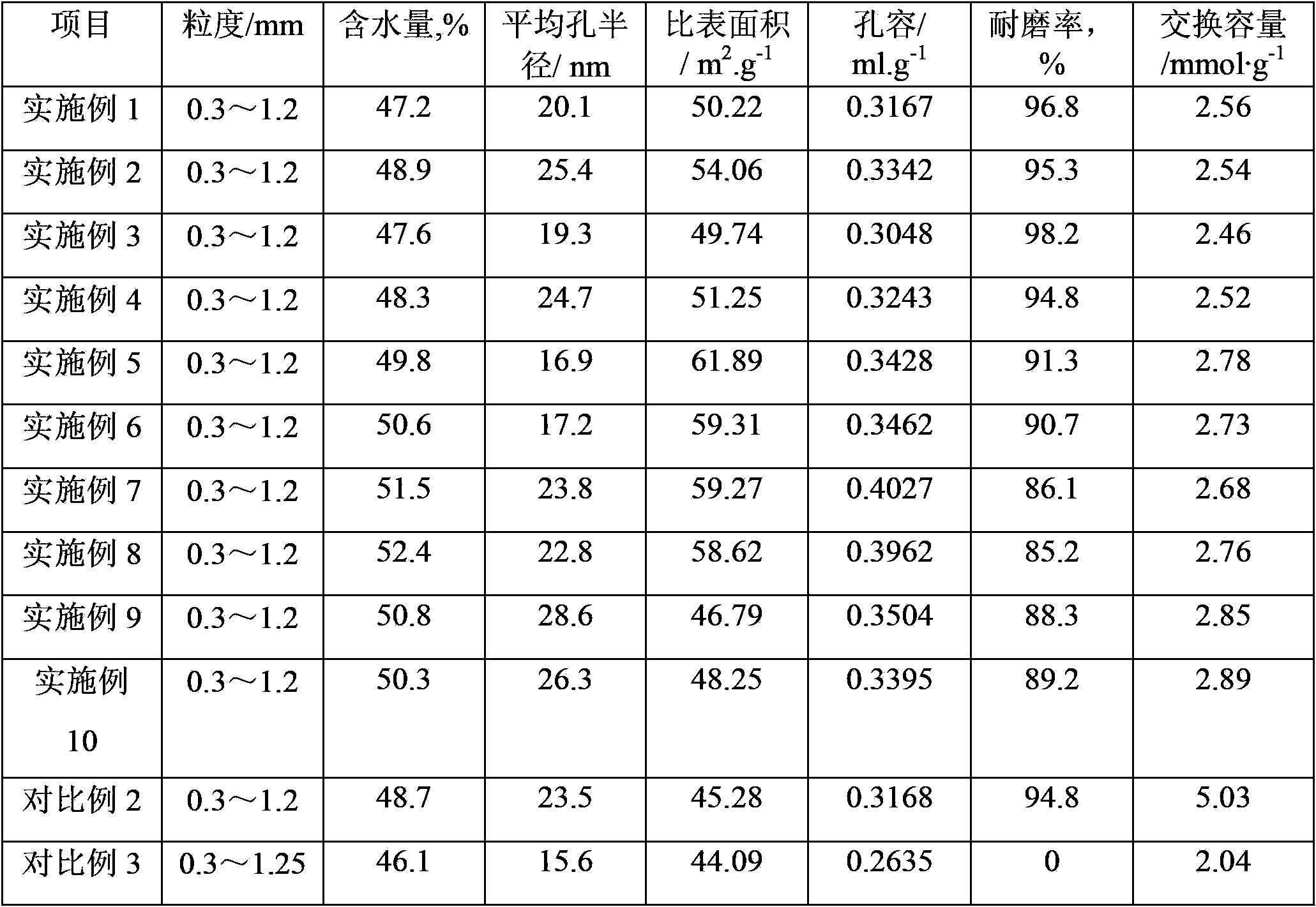
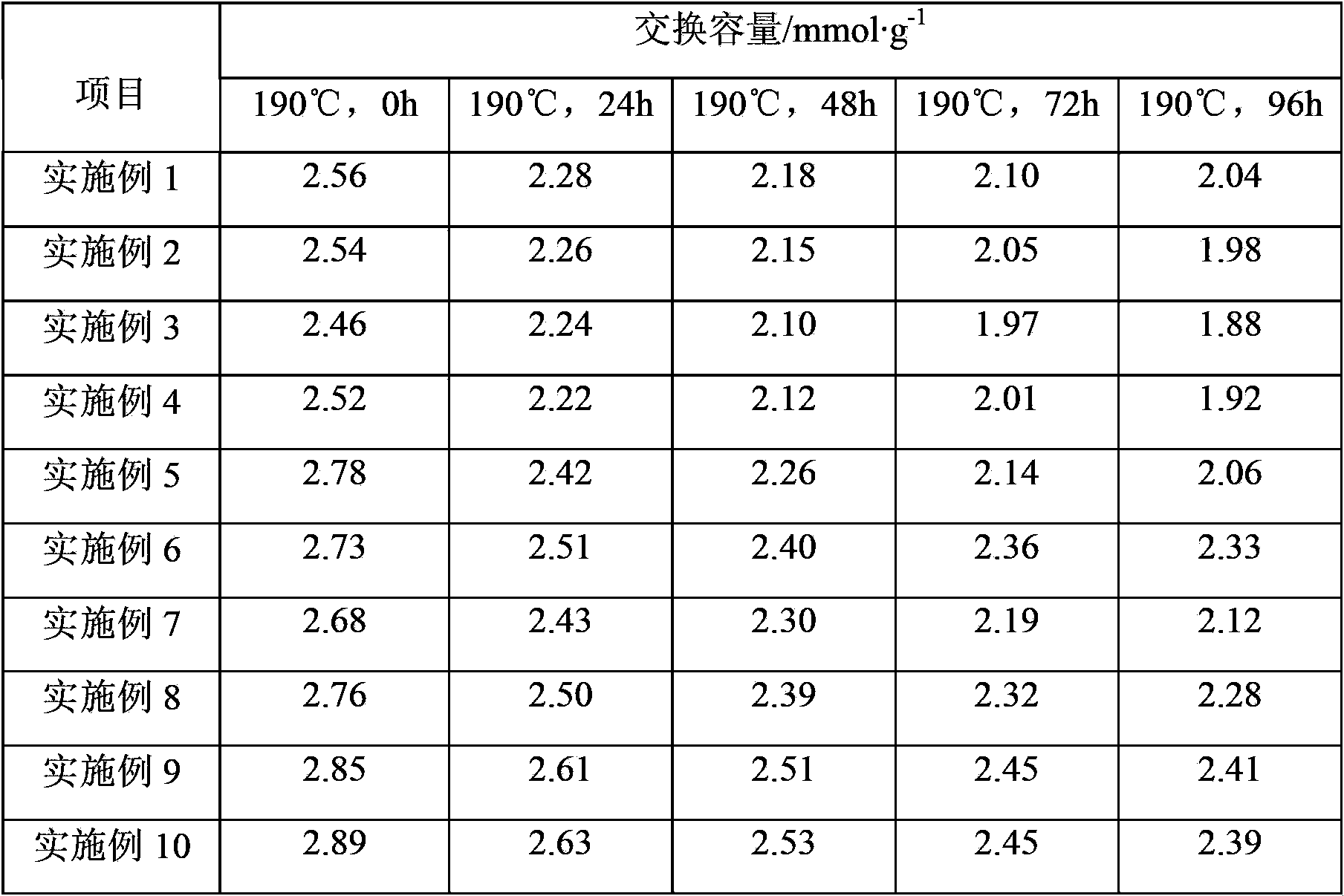
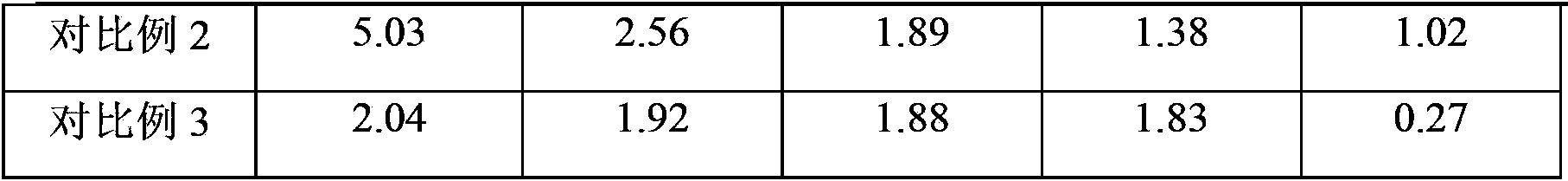
Embodiment 1
[0040] Polymerization: In a 1000mL four-necked flask equipped with a stirring, reflux condenser and thermometer, add 550g of water, 0.7g of polyvinyl alcohol with an alcoholysis degree of 70%, and 0.1g of hydroxypropyl methylcellulose, heating and stirring, It dissolves completely and forms a homogeneous aqueous solution. Warm up to 45℃, add 4g of methine blue aqueous solution with a mass concentration of 0.4%, then add 100g of styrene (styrene content ≥99%), 75g of divinylbenzene (divinylbenzene content of 50%), 44g Liquid paraffin, 0.8g tert-butyl peroxyneodecanoate, 0.4g tert-amyl peroxypivalate, the organic phase mixture, adjust the appropriate stirring speed, heat to 65℃, react at constant temperature for 2 hours, and then heat to 80 ℃, constant temperature reaction for 4 hours. The reaction product was filtered, and then placed in 3 times the volume of tap water and stirred for 5 minutes, washed the residual dispersant, filtered, so washed and filtered three times, and d...
Embodiment 2
[0055] Polymerization: In a 1000mL four-necked flask equipped with stirring, reflux condenser and thermometer, add 450g water, 2.6g gelatin, 0.3g polystyrene-maleic anhydride ammonium salt, heat and stir to make it completely dissolved and form a uniform Add 1mL of 0.4% methine blue aqueous solution and 12g potassium chloride. Heat to 45℃, add 104g styrene (styrene content≥99%), 96g divinylbenzene (divinylbenzene content 50%), 60g 36# white oil, 1.0g lauryl peroxide and 0.6g For the organic phase mixture composed of benzoyl peroxide, adjust the appropriate stirring speed, heat up to 65°C, react at a constant temperature for 3 hours, then heat up to 85°C, react at a constant temperature for 4 hours. The reaction product was filtered, and then placed in 3 times the volume of tap water and stirred for 5 minutes, washed the residual dispersant, filtered, so washed and filtered three times, and dried at room temperature (water content ≤ 3%) to obtain a dry copolymer 230g white ball...
Embodiment 3
[0062] Polymerization: styrene-divinylbenzene copolymerization was carried out in the same way as in Example 1, except that 1.16g tert-butyl peroxyneodecanoate was used as the initiator instead of 0.8g tert-butyl peroxyneodecanoate and 0.4g peroxide A mixture of tert-amyl pivalate. 198g of dried white copolymer balls were obtained, of which the particle size ranged from 0.3mm to 1.0mm, accounting for 89.6%.
[0063] Porogen extraction: The same method as in Example 1 was used to perform porogen extraction on 100 g of white copolymer balls, except that the number of solvent extractions was increased from 20 to 25. 77.6g of dry copolymer white balls with suitable pore structure were obtained, and the content of volatile matter was less than 1%.
[0064] Bromination: In a 5L enamel kettle, add 500g of dry copolymer white balls with suitable pore structure, 2500mL of dichloroethane, and 32g of iron powder, stir and swell for 3 hours, and use an ice water bath to reduce the temperature...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| water content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com