A kind of preparation method of compound microecological preparation effervescent tablet
A technology of compound microecology and effervescent tablets, which is applied in the direction of pharmaceutical formulations, drug combinations, and medical preparations containing active ingredients, etc. It can solve the inconvenience of storage and transportation for users, it is difficult to ensure accurate measurement of animals, and reduce the number of viable bacteria To achieve the effect of improving feed utilization, increasing convenience and reducing stress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
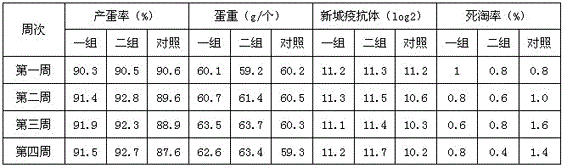
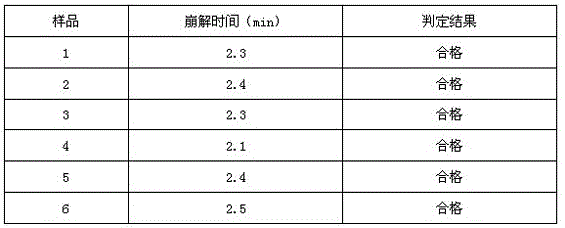
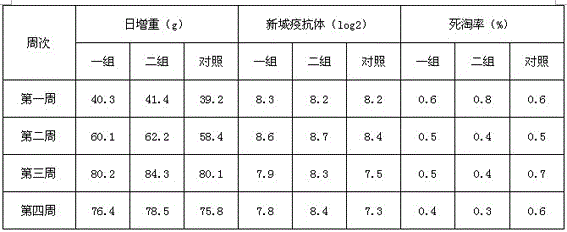
Examples
Embodiment 1
[0033] Embodiment 1: the preparation of probiotics
[0034] (1) Preparation of seeds for production
[0035] Culture of lactic acid bacteria
[0036] Preheat the MRS liquid medium to 37°C, inoculate the lactic acid bacteria seed liquid at 2% inoculation amount, and culture it at 37°C for 18-24 hours, harvest the bacterial liquid and store it at -4°C for later use.
[0037] Culture of Bacillus
[0038] Preheat the Bacillus culture medium to 37°C, inoculate the Bacillus seed solution with 1% inoculation amount, and culture it at 37°C for 18-24 hours, harvest the bacteria solution and store it at 4°C for later use.
[0039] The lactic acid bacteria are: one or more of Bifidobacterium bifidum, Lactobacillus lactis, Lactobacillus buchneri, and Bifidobacterium longum.
[0040] The bacillus is: one or more of Bacillus licheniformis, Bacillus coagulans and Bacillus subtilis.
[0041] Described MRS liquid culture medium is:
[0042] Peptone 10.0g, beef extract 10.0g, yeast extract...
Embodiment 2
[0045] Embodiment 2: Preparation of compound Chinese medicine polysaccharide and Chinese medicine flavone
[0046] a. According to 20 parts of Salvia miltiorrhiza, 15 parts of licorice, 20 parts of ginkgo biloba, 20 parts of astragalus, 15 parts of ginseng, 20 parts of Codonopsis pilosula, and 20 parts of wolfberry, weigh each Chinese medicine raw material, chop it, wash it, soak it in cold water overnight, and then add Purified water with 15 times the weight of the raw material, in a water bath to 90°C, and kept at 90°C, boiled for 2 hours, stirring every 10 minutes during the boiling process;
[0047] b. Discard the dregs of the traditional Chinese medicine in a, collect the medicinal liquid, and centrifuge at 10,000 rpm for 10 minutes after cooling at room temperature, discard the precipitate, and collect the supernatant;
[0048] c. The supernatant in b is subjected to alcohol precipitation, the precipitation is the crude Chinese medicine polysaccharide, and the supernatan...
Embodiment 3
[0054] Embodiment 3: preparation of lyoprotectant
[0055] Each 1000ml phosphate buffer solution contains the following components: 40g of Chinese medicine polysaccharides, 30g of Chinese medicine flavonoids, 8g of vitamin combination (vitamin C1.6g, vitamin E0.8g, vitamin D30.6g, vitamin B12.2g, vitamin K1.6g, vitamin B21. 2g), gelatin 5g, sucrose 50g; or every 1000ml of phosphate buffer contains the following components: Chinese medicine polysaccharide 70g, Chinese medicine flavonoids 40g, vitamin combination 7g (vitamin C1.4g, vitamin E1.75g, vitamin D30.7g, vitamin B11. 4g, vitamin K0.7g, vitamin B21.05g), gelatin 6g, sucrose 60g. Described Chinese medicine polysaccharide and Chinese medicine flavonoid are obtained by embodiment 2.
[0056] Preparation method: Accurately weigh each component, dissolve in 1000ml of phosphate buffer solution, filter and sterilize through a 0.22um filter membrane under sterile conditions, and store at 4°C for later use.
[0057] Phosphate b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com