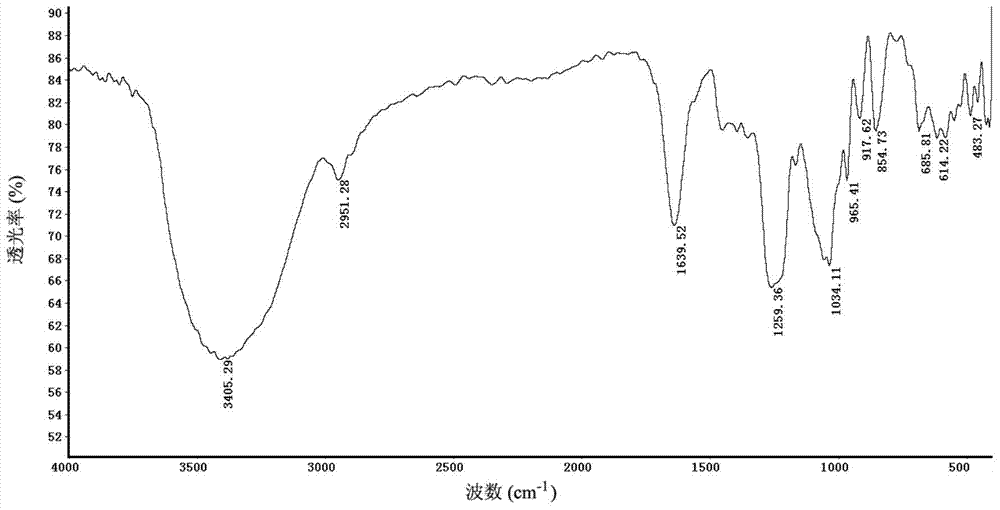
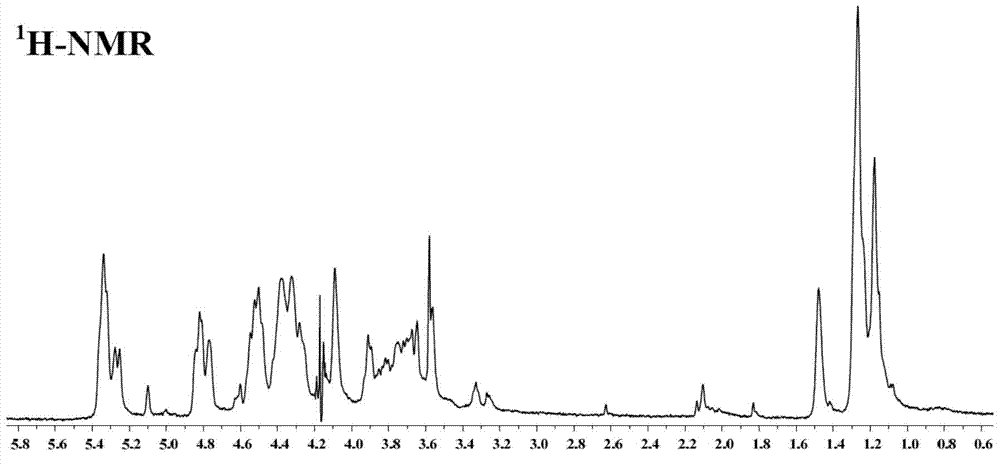
Fucosan sulphate, preparation method thereof, and application of fucosan sulphate in preparing anti-influenza virus medicine
A fucoidan sulfate, anti-influenza virus technology, applied in the directions of antiviral agents, pharmaceutical formulations, medical preparations containing active ingredients, etc., to achieve the effects of strong inhibitory effect, simple preparation process and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The preparation method of fucoidan sulfate provided by the present invention comprises the following steps:
[0038] (1) Hot water extraction and dilute acid precipitation: extract brown algae powder after degreasing treatment with hot water, combine the extracts, precipitate with dilute hydrochloric acid, and centrifuge. The hot water extraction temperature is 70-90° C., and the pH of the dilute acid precipitation solution is 1-3.
[0039] (2) Calcium chloride precipitation: add 1 to 3 mol / L calcium chloride aqueous solution to the supernatant after (1) centrifugation, take the supernatant after centrifugation, concentrate, dialyze, precipitate with ethanol, and obtain fucoidan sulfate after drying .
[0040] (3) DEAE-Cellulose purification: Dissolve the fucoidan sulfate obtained in (2) in water, use sodium chloride aqueous solution as the mobile phase (0<sodium chloride concentration≤2mol / L), and undergo DEAE-Cellulose anion exchange Resin separation, sulfuric acid-...
Embodiment 1
[0043] Embodiment 1: Preparation of fucoidan sulfate ester
[0044] 1. Extract the defatted kelp powder in 80°C water bath for 3 to 5 times, combine the extracts, cool to room temperature, adjust the pH of the extracts to 1 to 3 with dilute hydrochloric acid, centrifuge, and collect the supernatant.
[0045] 2. Stir and add 3mol / L of CaCl to the supernatant prepared in step 1 2 Aqueous solution, until the precipitation no longer occurs, centrifuge to take the supernatant, transfer the supernatant to a dialysis bag with a molecular weight cut-off of 7000D, dialyze until the conductivity of the dialysis fluid no longer changes, concentrate the fluid in the dialysis bag under reduced pressure, and vacuum freeze-dry Get fucoidan sulfate (KCP).
[0046] 3. Dissolve the fucoidan sulfate prepared in step 2 with distilled water, then pass through DEAE-Cellulose anion exchange resin for further purification, and use NaCl aqueous solution (0<sodium chloride concentration≤2mol / L) as elu...
Embodiment 2
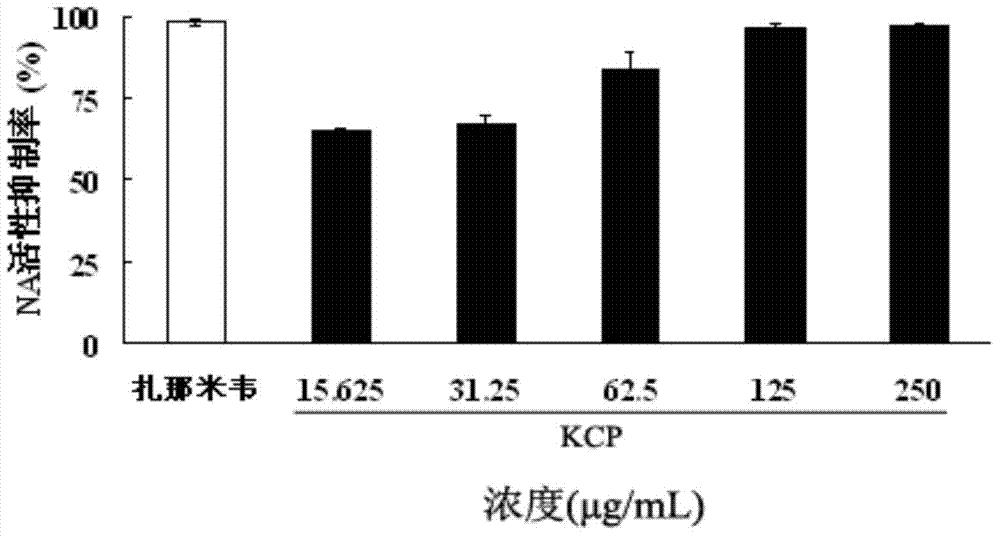
[0050] Embodiment 2: the effect of fucoidan sulfate against influenza A (H1N1) virus
[0051] 1. Fucoidan sulfate inhibits NA activity of H1N1, H5N1 and H3N2 influenza viruses
[0052] The fucoidan sulfate (KCP), H1N1, H5N1 and H3N2 neuraminidase NA and NA-specific substrate MUNANA (4-methylumbelliferyl-N-acetyl-α-neuralminic acid) were co-incubated in MES buffer, Using 4MU (7-hydroxy-4-methylcoumarin), the product of the interaction between NA and MUNANA, can produce 460nm fluorescence under the excitation of 355nm incident wavelength, and calculate the inhibition of fucoidan sulfate (KCP) on NA activity by detecting the change of fluorescence intensity Rate (the test method is recorded in the literature Li Hanbing, etc. Acta Pharmacina Sinica, 2009, 44(2): 162-166.), with zanamivir as the positive control drug.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com